| TUD Organische Chemie | Immel | Publications | Papers | Abstract 22 | View or Print (this frame only) |
Kahee Fujita, Wen-Hua Chen, De-Qi Yuan, Yasuyoshi Nogami, Toshitaka Koga, Toshihiro Fujioka, Kunihide Mihashi, Stefan Immel, and Frieder W. Lichtenthaler
Tetrahedron: Asymmetry 1999, 10, 1689-1696.
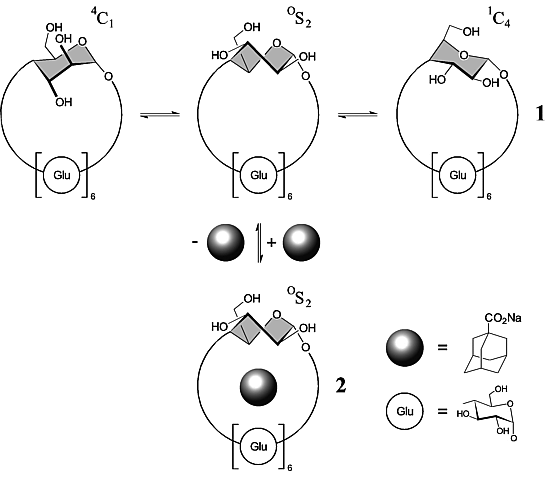
Mono-altro-β-cyclodextrin (1), a β-cyclodextrin with one of the seven glucose units configurationally changed to an altrose, is shown to be a flexible host undergoing, upon intracavity inclusion of adamantane-1-carboxylate, a distinct conformational change within its altropyranose geometry, thus representing an induced-fit model of binding rather than one following the rigid lock-and-key type pattern.
|
| Figure 1: Schematic representation of mono-altro-β-cyclodextrin (1) with its altropyranoid unit in 4C1, OS2, and 1C4 conformation, respectively, and its adamantanecarboxylate inclusion complex 2 in which the altrose residue is induced to preferentially adopt the OS2 form. |
Additional Graphics: Mono-altro-Cyclodextrins