| TUD Organische Chemie | Immel | Publications | Papers | Abstract 25 | View or Print (this frame only) |
S. Immel
Proceedings of the 10th Internat. Symp. on Cyclodextrins (Ed.: J. Szejtli), Mia Digital Publ., Ann Arbor, Michigan, 2000, pp. 18- 23.
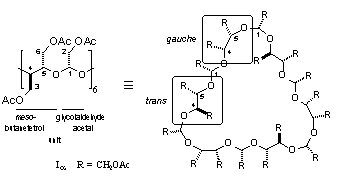
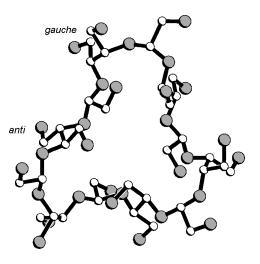
Kandra et al. reported the β-CD-derived title compound as its amorphous, chiral ([α]D = -0.1) per-O-acetate (Iβ),[1] suggesting it to be impure. Similarly, we have elaborated the periodate oxidation of α-, β-, and γ-CDs, CDs, followed by borohydride reduction, to give the respective 30-, 35-;, and 40-memebered cyclic crown acetals, which were isolated and characterized in the form of their achiral crystalline per-O-acetates (Iα - Iγ). X-Ray analysis of these compounds revealed their unique topology: in Iα with approximate C3 symmetry, three acetoxyethylidene units are located inside the macro-ring and the other three outside. Of the six acetoxy-meso-butanetetrol units, three are in a gauche and the other three in a trans arrangement, alternatively (Fig. 1). The macrocyclic frames of Iβ and Iγ have extra looping parts, but are in almost the same conformation as Iα.
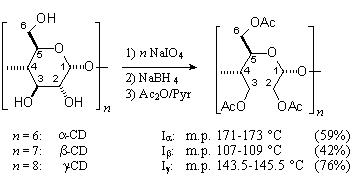 |
Fig. 1 (below). Conformation of Iα in the crystal with one meso-butanetetrol gauche- and trans-conformation labeled (cf. formula on the left; all acetyl groups and hydrogen atoms omitted for clarity). | |
 |
 |