| TUD Organische Chemie | Immel | Publications | Papers | Abstract 38 | View or Print (this frame only) |
F. W. Lichtenthaler, T. Weimer, and S. Immel
Tetrahedron: Asymmetry 2004, 15, 2703-2709.
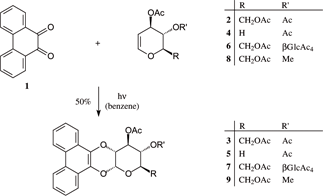
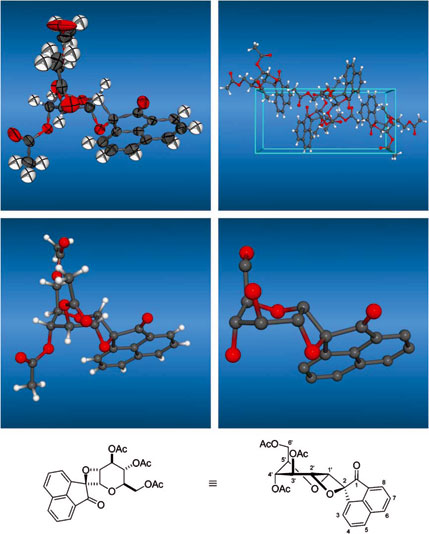
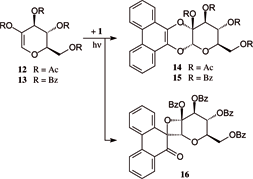
Photocycloadditions of phenanthrenequinone 1 and acenaphthenequinone 17 to 3,4,6-tri-O-acetyl-D-glucal proceeded with distinctly different regioselectivities: 1 preferentially adds with both carbonyl oxygens to give the [4+2] cycloadduct, a 1-O,2-O-annelated a-D-glucoside (50% [Chem. Ber. 1952, 85, 531]), while 17 reacts with only one carbonyl group to exclusively yield the [2+2] addition product, a 1-C,2-O-oxetano-a-D-glucoside (86%). 2-Hydroxyglucal esters 12 and 13 also undergo photoadditions with 1 to give the cis-1,4-dioxane-fused cycloadducts 14 and 15, whilst 17 fails to react. Structural and configurational assignments rest on NMR data and an X-ray analysis of spiro-pyranooxetane 18.
 |
 |
 |