| TUD Organische Chemie | Immel | Tutorials | Orbitals | Hybrid | View or Print (this frame only) |
Different hybrid orbitals, their formation by mixing various atomic orbitals, and their shapes are discussed here.
These hybrid orbitals include the common sp-, sp2-, sp3-orbitals frequently used in organic chemistry,
as well as hybrid orbitals including d-functions. The latter are particularly useful for describing the geometries and shapes of
transition metal complexes in the field of inorganic chemistry.
All graphics shown on this page were created using the MolArch+ program and POVRAY Persistence of Vision Raytracer. Hybrid Orbitals were generated from the pure hydrogenic wave functions of the corresponding atomic orbitals; Cartesian wave functions were taken from The Orbitron Gallery of Atomic Orbitals and Molecular Orbitals. Links are provided for still images as well as to 3D-models (VRML file format, which requires a plugin appropriate for viewing these file type to be installed). |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Linear Combination of Atomic Orbitals (LCAO) is a mathematical method to construct a new set of orbitals - the hybrid orbitals - by mixing appropriate atomic orbitals (AOs).
These hybrid orbitals are useful to account for the "true" geometry of molecules and the directionality of their constituting bonds (e.g. the tetrahedral geometry of methane).
Here n atomic orbitals (with their wave functions φ1, φ2, ..., φn) are used to construct n hybrid orbitals (ψ1, ψ2, ..., ψn) through a linear combination, where the coefficients c1,1, c1,2, ..., cn,n are normalization constants that must fulfill some requirements:
An atomic orbital (or hybrid orbital) is normal if the integrals ∫ φnφn δτ = 1.0 (or ∫ ψnψn δτ = 1.0 ) - this is the integral over the electron density (which is proportional to φ2 or ψ2) over the entire space ( ∫ δτ) must be equal 1.0, or in other words, the probability of finding any electron anywhere in the space must be 100%. Furthermore, all hybrid orbitals constructed this may must be orthogonal to each other, i.e. the integrals ∫ φnφm δτ = 0.0 for all n ≠ m - meaning that all possible pairs the newly formed hybrid orbitals must have a zero-overlap in total. The most common hybrid orbitals in use are the 2sp, 2sp2, and 2sp3 orbitals of carbon (used in organic chemistry to the describe the linear arrangement of bonds in alkines, the trigonal-planar geometry in alkenes and the tetrahedral form in alkanes), or the 3sp3dz2 (five AOs yield five hybrid orbitals) and 3sp3d2 (six hybrid orbitals) to describe the trigonal-bipyramidal and octahedral geometries of transition metal complexes. Below, examples for the different hybrid orbitals and visualizations of their shapes are given. All orbitals plots on this page are the 90% probability contours of the corresponding electron density (ψ2) with colors indicating opposite signs of the wave function (&psi). Please note, that hybrid orbitals are a powerful tool to describe the geometry and shape of molecules and metal complexes, yet in "real" molecules their significance may be debated. In "real" cases, someone has to refer more realistically to molecular orbitals instead. This page should give an overview on different geometries of hybrid orbitals, and the consequences for the shape of molecules. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2sp-Orbitals results from linear combination of a single 2s-orbital with one 2p-orbital (e.g. px here; check with conditions for normalization constants above, and note that different colors
in the orbital plot correspond to opposite sign of the wave function):
Click the above images to obtain enlarged views of the orbitals, respectively. Please note, that for the sp-orbitals, the common text-book representation places the nucleus at the center between the minor and the major lobe, which in fact is not correct. As obvious from these graphics, the nucleus is embedded in the minor lobe, off the nodal plane of the sp-orbitals.
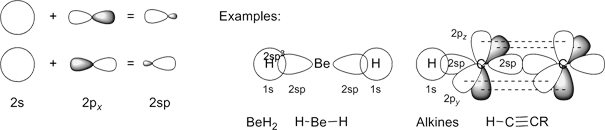 Of course, BeH2 in the solid state is not a monomeric molecule, but forms polymers of the form [BeH2]n. Shapes similar to the 2sp-orbitals (2s + 2p-orbitals) presented here, are found for the 3sp-orbitals and 4sp-orbitals which constructed from 3s + 3p- and 4s + 4p-orbitals, respectively. Yet, as expected the number of nodal planes increases with increasing principal quantum number. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The following links provide some animations on the mixing of a 2s-orbital with a 2px-orbital to form the corresponding 2sp-hybrid orbital. All animations are MPEG-movies,
and an external player is required (I prefer QuickTime over the Windows Media Player as yields a higher quality, the Media Player suffers from some MPEG-playback artifacts).
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The linear combination of a 2s-orbital with two 2p-orbitals results in 2sp2-orbitals (e.g. 2s-orbital plus px and py; check with conditions for normalization constants above, and note that different colors
in the orbital plot correspond to opposite sign of the wave function):
As for the sp-type hybrid orbitals discussed above, the common text-book representation of sp2-orbitals erroneously places the nucleus at the center between the minor and the major lobe, which is incorrect. As obvious from these graphics, the nucleus is embedded in the minor lobe, off the nodal plane of the sp2-orbitals.
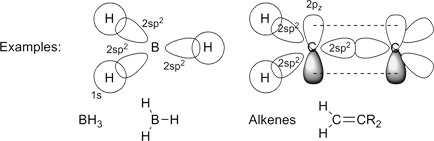 Note, that BH3 usually forms dimers of the type B2H6, and may not occur as an isolated molecule. Similar to the 2sp2-orbitals (2s + 2px,y-orbitals), hybrid orbitals can be formed from 3s + 3px,y- (3sp2) and 4s + 4px,y-orbitals (4sp2). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The linear combination of a 2s-orbital with all three 2p-orbitals (px, py, and pz) yields four 2sp3-Orbitals
(check with conditions for normalization constants above, and note that different colors in the orbital plot correspond to opposite sign of the wave function):
As for the sp-type and sp2-hybrid orbitals discussed above, for the sp3-orbitals the nucleus is embedded in the minor lobe, off the nodal plane.
 Similar to the 2sp3-orbitals (2s + 2px,y,z-orbitals), hybrid orbitals can be formed from 3s + 3px,y,z- (3sp3) and 4s + 4px,y,z-orbitals (4sp3). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Compounds with third row atoms (and above, including transition metals) often involve hybrid orbitals with d-functions. The following table provides an overview on the most
commonly used hybrid orbitals with together with the resulting geometrical shapes:
See the table below for some examples of transition metal complexes and their geometries. Click the hybrid designations to view images of the corresponding orbitals.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||