| TUD Organische Chemie | Immel | Tutorials | Orbitals | Hybrid | Orbitals 3sp3-3d | View or Print (this frame only) |
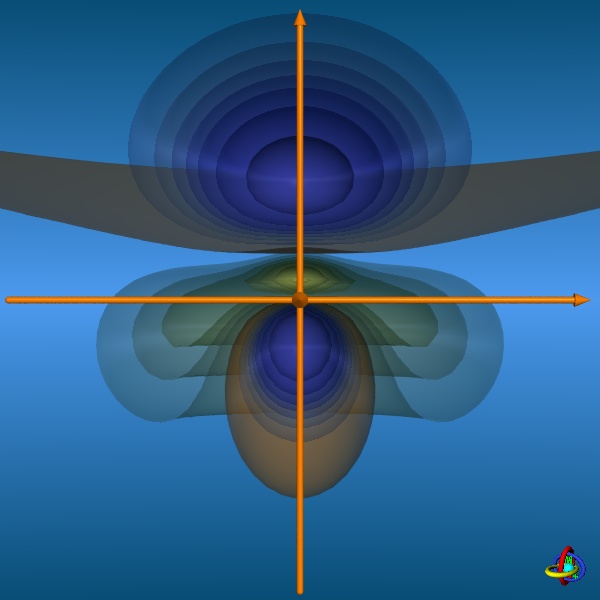
The linear combination of a 3s-orbital with three 3p-orbitals (3px, 3py, and 3pz) and one 3d-orbital (3dz2)
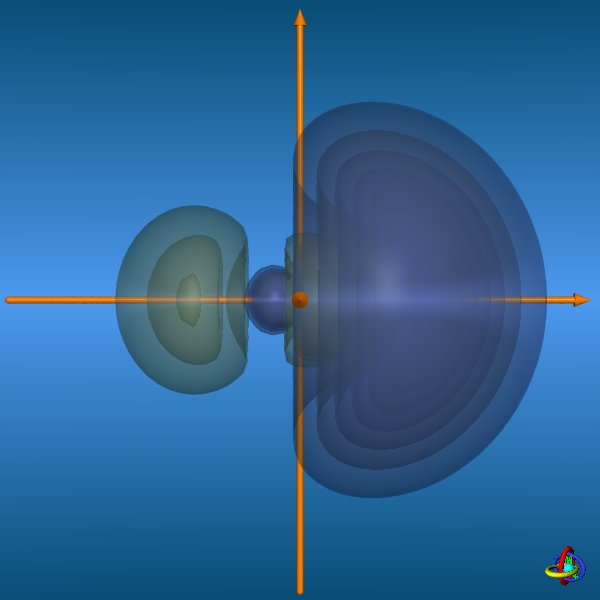
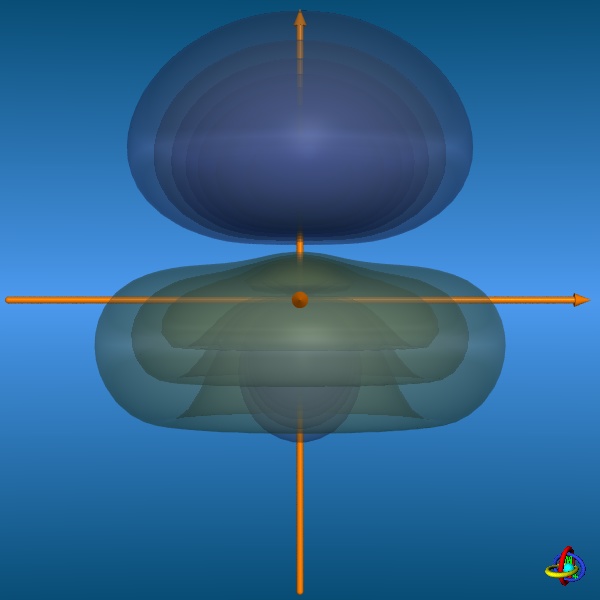
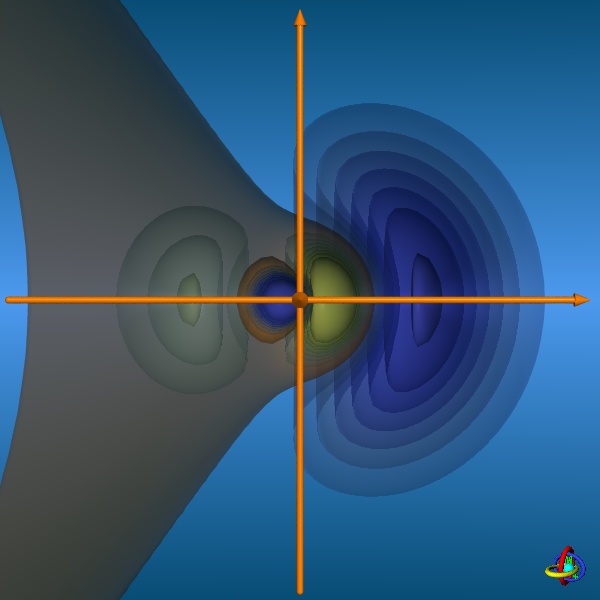
results in five sp3d-orbitals. These orbitals are special inasmuch the equatorial and axial positions are not equivalent, but differ in their shapes.
The equatorial orbitals actually represent a sp2-type hybrid (3s + 3px + 3py) with no d-character, whereas the axial orbitals
are pd-hybrids (3pz + 3dz2) without s-contribution (see the wave functions below).
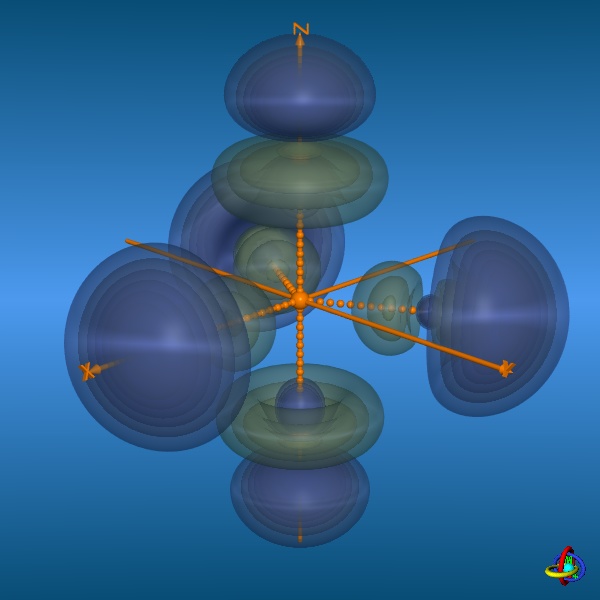
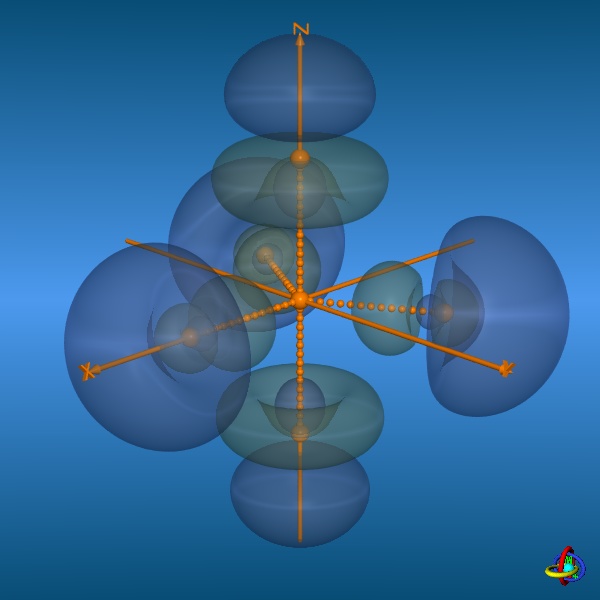
The images below provide an illustration of the probability contours (90-25% probability level of the electron density, i.e. ψ2) and the nodal planes of a sp3d-orbital (actually not "planar" planes; ψ = 0), where different colors indicate regions of opposite sign in ψ. In the column on the right, all five sp3d-orbitals and their trigonal-bipyramidal arrangement are displayed, but note that the orbitals have been moved away from the center towards the outside to facilitate visualization (the orange spheres represent the original location of the nucleus). Click on the images to enlarge view or see the VRML 3D-model. |
|||
All graphics shown on this page were created using the MolArch+ program
and POVRAY Persistence of Vision Raytracer. Hybrid Orbitals were generated from the
pure hydrogenic wave functions of 3s- and 3p-orbitals; Cartesian wave functions were taken from
The Orbitron Gallery of Atomic Orbitals and Molecular Orbitals.
|
|||
|
|
|||