| TUD Organische Chemie | Immel | Tutorials | Orbitals | Molecular | Ethyne | View or Print (this frame only) |
The molecular orbitals (MOs) of molecules can be constructed by linear combination of atomic orbitals (LCAO).
Though the exact Schrödinger equation is unsolvable for many electron systems such
as molecules, the solution can be numerically approximated by ab initio or density functional (DFT) theory.
This page gives an overview on the molecular orbitals of ethyne calculated by DFT methods using a B3LYP/6-311++G** basis set. All MO representations are 90% or 90-25% iso-contour probability surfaces of the electron density (ψ2), i.e. they resemble the spatial volume around the nuclei of the molecule in which the electrons are found with the corresponding certainty. The different colors (yellow and blue) represent regions with opposite sign of the wave function ψ; nodal planes (not necessarily real "planar" planes) were ψ passes through zero and changes sign are indicated in orange.
Click on the small images below with blue background below to obtain an enlarged view - the images with black background provide links to the corresponding 3D-models (VRML-type models); these links will open in a new window. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
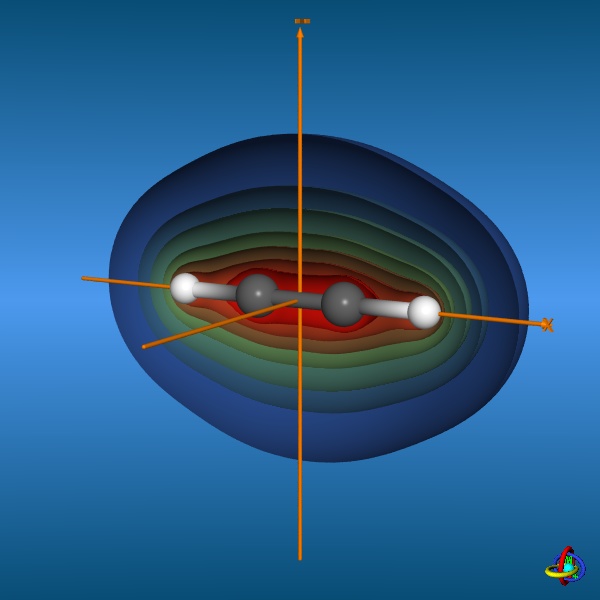
The total electron density (clipped 99, 95, 90, 80, 70, 60, and 50% iso-density contours depicted on the right) render the molecule with its characteristic shape
(note the different iso-contour values of the MO orbitals and the total electron density contours).
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
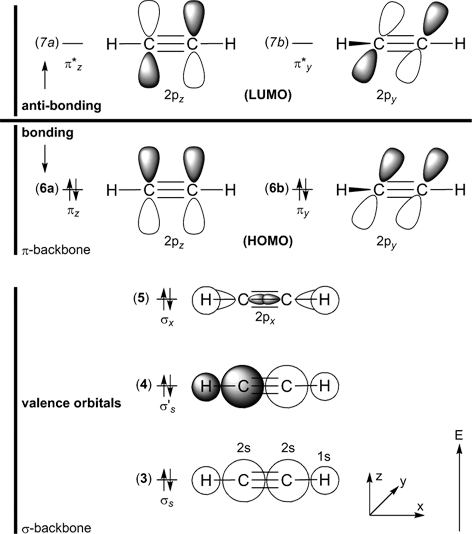
Below on the right, the schematic drawing indicates the major contributions of atomic orbitals (AOs) to the molecular orbitals (MOs) of ethyne.
With increasing energy of the orbitals (from bottom to top), the number of nodal planes (not necessarily real "planar" planes) increases and the symmetry decreases.
For ethyne, there are 14 electrons (C2H2, 2×6 + 2×1 = 14 electrons) and seven occupied orbitals. The lowest-energy orbitals are the 1s-carbon core orbitals
(bonded and anti-bonded combination, with a total of four electrons; MO no. 1 and 2) are not shown here, the remaining five valence MOs are constructed from the carbon
2s,
2px,
2py,
2pz, and hydrogen
1s-orbitals.
The highest occupied molecular orbitals (HOMOs) are two-fold degenerate, i.e. both orbitals 6a and 6b have equal energy.
The same applies to the lowest unoccupied molecular orbitals (LUMOs) 7a and 7b.
Further informations on AOs are available from the gallery of hydrogenic orbitals and hybrid orbitals.
The thumbnail images on the left provide access to enlarged graphics as well as 3D-models (VRML) of the orbitals, respectively. All images and models are scaled relative to each other, and their sizes can be compared directly. Only the orbitals made up from the valence AOs are displayed. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Notes: a) Orbital number (valence orbitals only, see scheme on the right, degenerate orbitals with equal energy are denoted by same numbers; numbers of unoccupied orbitals are in italics); b) Label (symmetry descriptor in parenthesis); c) Nodal planes (ψ = 0.0); d) 90% Probability contours of MO electron density (ψ^2); e) 90, 80, 70, 60, 50, 40, and 25% Probability contours; f) 3D-Models require a VRML plugin to be installed (large files with sizes between 500-2800 KBytes). |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
For a more detailed description of atomic orbitals see the corresponding gallery of orbitals.
All graphics and iso-contour surfaces shown on this page were created using the MolArch+ program
and POVRAY Persistence of Vision Raytracer. Electron densities were calculated
on three dimensional grids for the corresponding molecules using the JAGUAR program.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||