 MOLecular ARCHitecture+ - MolArch+ Movies MOLecular ARCHitecture+ - MolArch+ Movies
|
This page gives an overview on different types of molecular animations
that were generated using the MolArch +
program.
|
 |
 Claisen Rearrangement Claisen Rearrangement |
|
 Cope and Claisen Rearrangements Cope and Claisen Rearrangements |
The Cope rearrangement is a concerted thermal process by which 1,5-hexadienes (X = -CH 2-) rearrange. Closely related thereto
is the Claisen rearrangement (X = -O-) of allyl vinyl ether to form 4-pentene-al. Computational studies indicate that
both mechanisms involve highly delocalized aromatic transition states with six electrons. The animations display
either the chair- or boat-type transition state geometries of both processes, the former being usually preferred over
the latter. For further details see
H. Jiao, P. von R. Schleyer, "Aromaticity
of pericyclic reaction transition structures: magnetic evidence.", J. Phys. Org. Chem. 1998, 11, 655-662.
Other examples for (degenerate) Cope rearrangements presented on this page are the valence isomers of
bullvalene, hypostrophene, barbaralane, semibullvalene, and homotropylidene; another similar process
is the disrotatory ring closure of 1,3,5-hexatrienes.
|
|
|
|
 Valence Isomers of Bullvalene Valence Isomers of Bullvalene |
Bullvalene may be considered the ideal case of a hydrocarbon with a fluctuating structure: through degenerate Cope
rearrangements more than 10!/3 (> 1.2 million) valence tautomers with identical constitution are produced
in which the cyclopropane ring can be located at any three carbon atoms that are adjacent. At -25°C the
proton NMR spectrum shows the signals of a single structure whereas at 100°C only one signal is
observed for all protons, indicating fast averaging over all equivalent positions.
|
The animations show parts of this rearrangement (front-, side- and top-view) during which the
C 6-C 7-C 8-fragment undergoes an apparent 360° full rotation.

|
|
|
 Valence Isomers of Hypostrophene Valence Isomers of Hypostrophene |
In analogy to bullvalene, hypostrophene is another example of a hydrocarbon (both have the
common formula C10H10) for which degenerate Cope rearrangements result in equivalence of all carbon atoms.
|
|
|
|
 Valence Isomers of Barbaralane Valence Isomers of Barbaralane |
Degenerate Cope rearrangements result in two equivalent forms of barbaralane (bullvalene in which one -CH=CH-
fragment has been replaced by a methylene group). At room temperature rapid interchange is present, though by about
-100°C this is slowed to a point where the NMR spectra are in accord with a single ("frozen") structure.
|
|
|
|
 Valence Isomers of Semibullvalene Valence Isomers of Semibullvalene |
Semibullvalene (barbaralane in which the methylene group has been removed) probably shows the lowest energy barrier
of all compounds undergoing degenerate Cope rearrangements, since not only at room temperature but even at
-110°C rapid interconversion between two equivalent forms is observed.
|
|
|
|
 Valence Isomers of Cyclooctatetraene Valence Isomers of Cyclooctatetraene |
This example shows the interconversion of cyclooctatetraene and bicyclo[4.2.0]octa-2,4,7-triene.
Both valence isomers coexist in an equilibrium with relative proportions of 99.99% and 0.01%, respectively (however,
on bromination of cyclooctatetraene the 7,8-dibromo-bicyclo[4.2.0]octa-2,4,7-diene is formed predominantly).
The animation shows the interconversion between both forms, and the changes in molecular geometry associated with
this rearrangement.
|
|
|
|
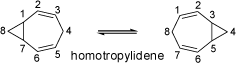
 Valence Isomers of Homotropylidene Valence Isomers of Homotropylidene |
Homotropylidene (bicyclo[5.1.0]octa-2,5-diene) undergoes a degenerate Cope rearrangement similar to
to the rearrangement of 1,5-hexadienes, which produces identical
structures. Nevertheless, these valence tautomers (or valence isomers) are quite different from resonance structures
(mesomeric forms): even though only electrons are shifted, the positions of the nuclei are not the same in both
structures. At room temperature the NMR spectrum is in accord with the structure of homotropylidene, whereas at 180°C
only an averaged set of signals is observed.
|
This indicates rapid interconversion (> 10 3/s) of both forms as visualized by the animations on the left. At -50°C
this process is halted almost completely.
|
|
|
 Isomerization between Diademane and Triquinacene Isomerization between Diademane and Triquinacene |
Consistent with the Woodward-Hoffmann rules, the thermal isomerization from diademane to triquinacene
is a concerted process with an experimental activation barrier of about 120kJ/mol in solution. Triquinacene
with three C=C double bonds in rigid positions has been considered as a possible neutral homoaromatic compound, but
it is not as its heat of formation provided no evidence of the expected homoaromatic stabilization.
However, the transition state of the isomerization is highly aromatic based on computational results. The animations
on the left visualize the diademane to triquinacene isomerization (all structures are of C3v symmetry).
|
|
|
|
|
|