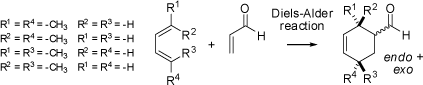
Diels-Alder reaction of the different 2,4-hexadienes (trans,trans-, cis,trans-, trans,cis-, or cis,cis-) with acroleine as
the dienophile result in racemic endo- or exo-products with respect to the position of the CHO-group.
The [4ps + 2ps] cycloaddition
is highly stereospecific and the geometry of the diene is fully retained
in the cyclohexene type products. Vice versa, the relative configuration of the cyclohexene
determines the configuration of the resulting diene in the reverse reaction (disrotatory motion). The course of the
Diels-Alder reaction is determined by the relative symmetry of the HOMO of the diene (cyclopentadiene) and the LUMO
of the dienophile (acroleine). The animations show the stereospecificity of the cycloaddition between all four
isomeric 2,4-hexadienes and acroleine.
|
Please note the increasing distortion due to steric crowdance and non-planar conformation of the diene in the order
trans, trans < cis, trans = trans, cis << cis, cis. At least for the latter
cis, cis-2,4-hexadiene the Diels-Alder reaction is not a favored process.
|