 MOLecular ARCHitecture+ - MolArch+ Movies MOLecular ARCHitecture+ - MolArch+ Movies
|
This page gives an overview on different types of molecular animations
that were generated using the MolArch +
program.
|
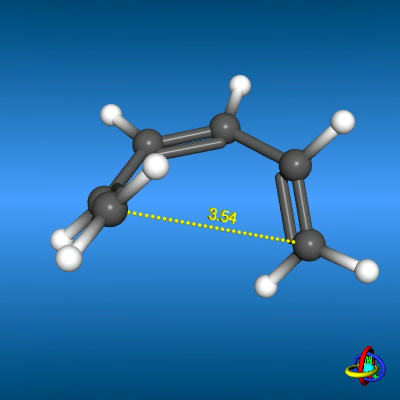 |
 Ring Closure of Hexatriene Ring Closure of Hexatriene |
|
 Antarafacial [2+2] Cycloaddition Between Cyclopentadiene and Methylketene Antarafacial [2+2] Cycloaddition Between Cyclopentadiene and Methylketene |
The thermal reaction of cyclopentadiene with ketenes yields in a [2+2]-cycloaddition cyclobutanones rather than
Diels-Alder type [4+2]-cycloadducts. Although thermal [2+2]-cycloadditions in which the
p-bonds of both reactants approach each other from the same side ("suprafacial",
[2ps + 2ps]) are symmetry forbidden, the
corresponding [2ps + 2pa] processes during which one
of the p-bonds is attacked from opposite sides ("antarafacial") are thermally allowed. This
mechanism is observed for ketenes or other linear molecules for which steric hindrance to such an antarafacial
approach is minimal. These reactions are stereospecific and for cyclopentadiene and methylketene
only the endo-product is formed. Most remarkably, the larger methyl group (compared to the smaller hydrogen
atom on the other side of the ketene) is forced into the more crowded endo-position in a
"masochistic steric" process.
|
This is induced by orbital symmetry (HOMO of the cyclopentadiene and LUMO of the
ketene), and the necessity to attack the p-orbitals from opposite sides of the ketene. The animations visualize the
molecular rearrangement and the orbital symmetry (basis set of atomic p-orbitals, not molecular orbitals) of
this concerted cycloaddition.
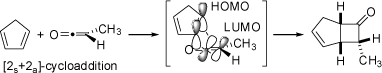
|
|
|
 Conrotatory Ring Opening of Cyclobutenes Conrotatory Ring Opening of Cyclobutenes |
Thermal ring opening of cyclobutenes proceed in a stereospecific manner, forcing the groups between which the
s-bond is broken into a conrotatory motion The resulting p-orbitals have the symmetry of the highest
occupied molecular orbital, HOMO, of the diene. Therefore cis-3,4-dimethyl-cyclobutene
produces cis, trans-2,4-hexadiene and vice versa. The Möbius aromatic transition state is
characterized by four delocalized p-electrons.
The animations on the left visualize the atomic motions during ring
opening and closure; the schematic p-orbitals shown are not molecular orbitals but a basis set of p-orbitals of
appropriate symmetry (HOMO of the diene, and the lowest unoccupied molecular orbital, LUMO, of the cyclobutene).
Data and transition state structures were taken from
http://www.bluffton.edu/~bergerd/classes/CEM311/examples/conrotate.html
|
|
|
|
 Disrotatory Ring Closure of Hexatrienes Disrotatory Ring Closure of Hexatrienes |
The thermal ring closure of 1,3,5-hexatrienes yields cyclohexadienes, a process in the course of which the
head groups of the triene must undergo disrotatory motion, the same applies to the reverse
ring opening reaction. Thus, trans, cis, trans-2,4,6-octatriene produces cis-5,6-dimethyl-1,3-cyclohexadiene only (both
terminal methyl groups end up on the same side of the six-membered ring) and vice versa. The animations visualize the
disrotatory motion of molecular fragments, which is required by the symmetry of the orbitals (highest
occupied molecular orbital, HOMO, of the triene) forming the s-bond between p-orbitals at both ends
of the p-system which are in "phase" (see animations of schematic drawings of atomic p-orbitals). Data and transition
state structures were taken from
http://www.bluffton.edu/~bergerd/classes/CEM311/examples/disrotate.html.
|
|
|
|
 Ring Closure Reaction of 1,3,5,7-Octatetraene Ring Closure Reaction of 1,3,5,7-Octatetraene |
The thermal ring closure reaction of 1,3,5,7-octatetraene, has eight delocalized electrons, and therefore it is another example
for a Möbius aromatic transition state (see also the conrotatory ring opening of cyclobutene above); for further details see
H. Jiao, P. von R. Schleyer, "Aromaticity of pericyclic reaction
transition structures: magnetic evidence.", J. Phys. Org. Chem. 1998, 11, 655-662.
|
|
|
|
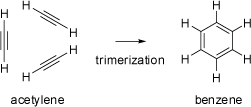
 Trimerization of Acetylene Trimerization of Acetylene |
At high temperatures (400-500°C) acetylene trimerizes to yield benzene, although the yield is low and
many other aromatic hydrocarbons are formed as side products (in the presence
of metal catalysts the trimerization and tetramerization may become the main reaction).
Based on the experimental heats of formation of benzene and acetylene, the trimerization of acetylene should be
extremely exothermic by about 600kJ/mol, but this thermally allowed reaction has a substantial activation barrier
(estimated by computational methods to about 200kJ/mol). This barrier arises from the energy required
to distort the three acetylenes to the transition state geometry, as well as electronic contributions dominated
by the closed-shell repulsions between filled orbitals. Nevertheless, the corresponding transition state of the
concerted cycloaddition is aromatic and of D3h symmetry. The animations on the left
visualize the transition between triple bonds and the aromatic ring system. The schematic orbital
drawings are not molecular orbitals, but only atomic p-orbitals of the acetylene units.
|
For details see e.g. R. D. Bach, G, J. Wolber, H. B. Schlegel,
"The origin of barriers to thermally allowed six-electron pericylcic reactions. The effects of HOMO-LUMO interactions
on the trimerization of acetylene.", J. Am. Chem. Soc. 1985, 107, 2837-2841; and H. Jiao, P. von R. Schleyer, "Aromaticity of
pericyclic reaction transition structures: magnetic evidence.", J. Phys. Org. Chem. 1998, 11, 655-662.
|
|
|
|
|