| TUD Organische Chemie | Immel | Tutorials | Hydrocarbons | Classes | View or Print (this frame only) |
This section gives examples for the structural variety of various classes of hydrocarbons.
Many molecular geometries were obtained from the Cambridge Crystallographic data file (CCDF) of solid-state structures or by simple force-field based geometry optimizations. The bond-lengths and angles do not necessarily correspond to the 'real' and correct values, but the molecular geometries shown on these pages nevertheless provide an growing pool of 3D information and may provide some 'ideas' on the shapes of molecules. |
|
||
Cycloalkanes with four or more carbon atoms in the ring are non-planar, but puckered ring systems which may exist in various low-energy
conformations. Five-membered rings are highly flexible, whereas cyclohexane-type structures predominantly adopt chair conformations.
Larger rings become increasingly more flexible with growing ring size.
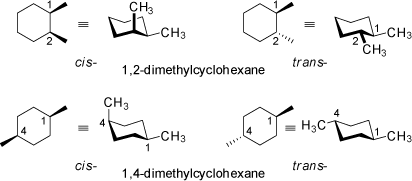
 The 3D structures of different 1,2- and 1,4-dimethyl-substituted cyclohexane derivatives visualize the energetically preferred conformations of these compounds. Trans-1,2-disubstituted cyclohexanes are chiral compounds which may be separated into two enantiomers (see also the 3D structures at 'Two or More Asymmetric Substituted Atoms').
 For some animations of the dynamic ring behavior of cyclopentane, cyclohexane, and substituted cyclohexanes see the chapter 'Ring Pseudorotation' in the MolArch+ - Movies section of this web-site.
|
|||
Small rings with three to seven carbon atoms allow for cis double bonds only, whereas larger rings may also accommodate trans double bonds.
Trans-cyclooctene is the smallest cyclic alkene with a trans double bond in a ring (see also the 'Bicyclic Compounds' below).
Although this cycloalkene is strained, it is a stable compound which exists in two separable enantiomeric conformations.
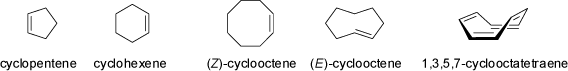 1,3,5,7-Cyclooctatetraene is not a planar, anti-aromatic ring, but a boat-shaped cyclic alkene with isolated double bonds. For further informations on the dynamic behavior and the 'Valence Isomers of Cyclooctatetraene' see the MolArch+ - Movies section of this web-site.
|
|||
Bicyclic compounds feature fused rings with two common atoms (usually carbon atoms, but also any other atom type) in their rings.
The ring systems may be strained, and some restrictions apply to the location of stable double bonds in these bicyclic structures.
According to Bredt's empirical rule, double bonds will not be formed at bridgehead positions as such structures have too much ring strain to be stable
(although some exceptions to this rule are known as "Anti-Bredt structures"). This rule originates from the impossibility
to align the p-orbitals of the p-bond parallel to each other at the bridgehead positions in these
geometrically constrained compounds. Thus, bicyclo[2.2.1]-2-heptene is a stable compound, whereas bicyclo[2.2.1]-1(2)-heptene is not stable
(see also the corresponding bicyclo[2.2.2]octanes). These preferences diminish with increasing ring size, and some exceptions may be observed
as highly reactive reaction intermediates.
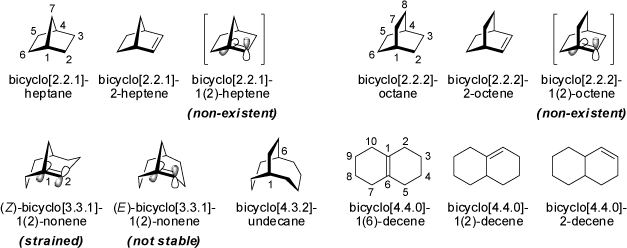 However, (Z)-bicyclo[3.3.1]-1(2)-nonene is a strained, but stable compound (the E-isomer is not stable) which may be regarded as a methylene-bridged derivative of trans-cyclooctene (see also the 'Cycloalkenes' above). Both compounds have about the same order of magnitude of internal ring strain energy. The isomeric bicyclo[4.4.0]decenes are known stable compounds featuring double bonds in cyclohexene type ring systems.
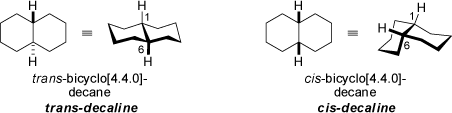 Both the stereoisomeric trans- and cis-bicyclo[4.4.0]decanes (trans-decaline and cis-decaline) feature two fused cyclohexane rings in chair conformations, respectively. The trans-form has two equatorial-equatorial linkages, whereas in the cis-compound two equatorial-axial linkages are realized. The trans-form is by 8.4 kJ/mol more stable than cis-decaline. In addition, trans-decaline is rather rigid, while the more flexible cis-structure undergoes interconversion between enantiomeric conformations.
|
|||
Spiranes (spira = pretzel) have only one atom (usually a quaternary carbon) as a common member of two rings. Some sesquiterpenes have been
identified to feature such a spiro-type annelation of ring systems. Characteristically, the planes of the two rings connected via their
common atom are aligned perpendicular to each other. Interestingly, spiranes may be chiral compounds when adequately substituted,
although they may not feature a chiral center, but a chiral axis only (see also 'Axial Chirality').
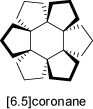
 The [6.5]coronane is a cyclic spiro-compound of very unusual symmetry (S6, see also 'Achiral Compounds').
 |
|||
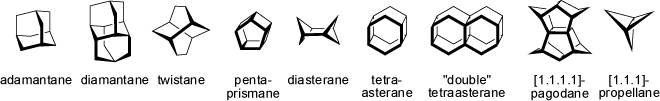
Polycyclic hydrocarbons with three or more fused ring systems adopt interesting shapes, with more or less strained ring systems. Adamantane features a
rigid, relaxed structure in which all cyclohexane rings adopt low-energy chair conformations (1-adamantyl derivatives are very bulky
substituents which are even larger and more congested than tert-butyl groups). In contrast, the cyclohexane rings of twistane are
fixed in less favorable skew-boat conformations. Amongst other polycyclic compounds of irregular shape (e.g. pagodanes and propellanes) some highly
symmetrical polyhedranes are shown (see 'Polyhedranes') below.
 |
|||
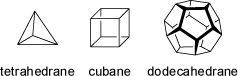
Polyhedranes are polycyclic hydrocarbons of the (CH)2n series having skeletons corresponding to the regular and semi-regular geometrical solids.
Amongst the regular Platonic shapes tetrahedrane (n = 2; as its tetra-tert-butyl derivative, decomposition at 135°C), cubane (n = 4; a highly volatile hydrocarbon) and
dodecahedrane (n = 10; with the very high symmetry Ih) have been synthesized and isolated.
 |
|||
The unsaturated hydrocarbons shown below are not fixed single isomers, but structures which undergo rapid interconversions of their
backbones through degenerate Cope-rearrangements. Bullvalene may be considered the ideal case of a hydrocarbon with a fluctuating
structure: through degenerate Cope rearrangements more than 10!/3 (> 1.2 million) valence tautomers with identical constitution are
produced in which the cyclopropane ring can be located at any three carbon atoms that are adjacent. For further informations and some animations of
the fluctuating structures see the chapter 'Cope Rearrangements' in the
MolArch+ - Movies section of this web-site.
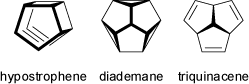
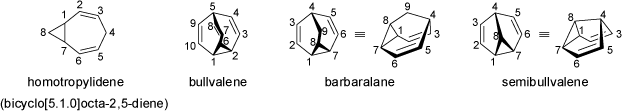 Other examples of rapid interconversions between valence tautomers for hydrocarbons are provided by the rearrangement of hypostrophene, or by the isomerization between diademane and triquinacene (see also the animations of 'Cope Rearrangements').
 |
|||
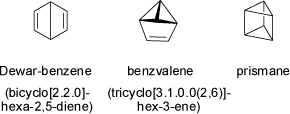
As examples for small bi- and polycyclic ring systems the highly strained valence isomers of benzene, i.e. Dewar-benzene, benzvalene, and prismane (all C6H6)
have been synthesized and characterized.
 |
|||
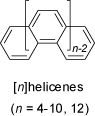
Helicenes are benzologues of phenanthrene. With four or more rings (tetrahelicene,
or [4]helicene) the compounds exist in two non-planar, but helical and enantiomeric forms, with C2 symmetry. In addition to the
structures of various helicenes of different ring size shown here, see also 'Helical Chirality')
and the 'Racemization of [8]Helicene' in the
MolArch+ - Movies section of this web-site for further informations.
 |
|||
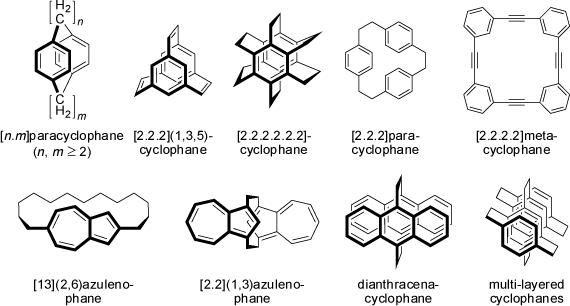
The term cyclophanes originally applied to compounds having two p-phenylene groups held face to face by -[CH2]n- bridges. It now designates compounds having
alternating arrangements of mancude-rings (rings formally having the maximum number of non-cumulative double bonds) systems or assemblies thereof,
and saturated or unsaturated chains which hold these mancude-ring rings together. There is a large variety of benzene (or other aromatic rings) derivatives connected via very short
or longer linkers, in which the p-systems are stacked face to face. In particular very sort linkages (e.g. ethylene bridges) may introduce significant
strain and distortions into the aromatic rings.
 |
|||
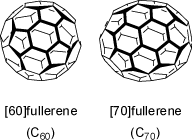
Although fullerenes are not hydrocarbons, but consist of carbon atoms only, they feature interesting shapes.
These compounds are composed solely of an even number of carbon atoms, which form a cage-like fused-ring polycyclic system with twelve five-membered rings
and the rest six-membered rings. The archetypal example is [60]fullerene, where the atoms and bonds delineate a truncated icosahedron. The larger
[70]fullerene is expanded by an additional ring of six-membered rings along the equator of its longer axis.
 |
|||