| TUD Organische Chemie | Immel | Tutorials | Symmetry | Point Group Determination | View or Print (this frame only) |
Determination of Point Group:
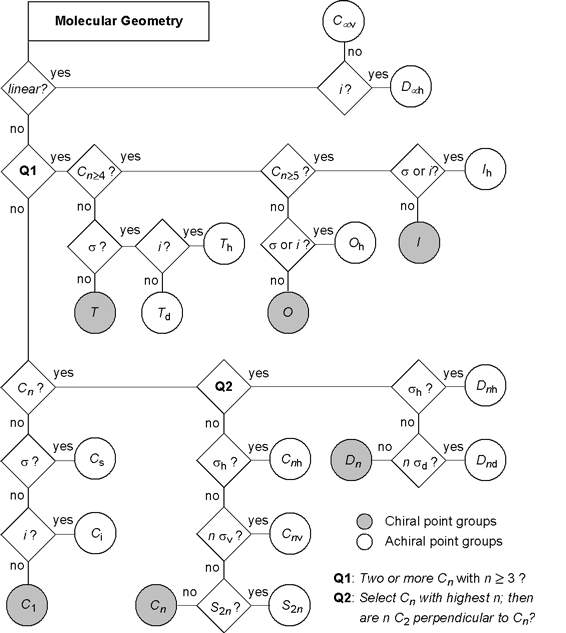
The flowchart below gives the accurate procedure to determine the point group a molecular geometry belongs to. Start from the top left and answer the questions indicated in the square boxes until you end up at a specific point group symbol. Please note, that different conformations of a given molecular structure may belong to different point groups depending on their symmetry properties. In this diagram, chiral point groups are marked by gray shading, molecules (or molecular geometries) belonging to these point groups must be chiral. All other point groups posses at least one rotary-reflection axis Sn of symmetry (including mirror planes σ = S1 or inversion centers i = S2), and thus the corresponding molecules must be achiral. For more details see the section 'Molecular Structures of Organic Compounds - Chirality' on this web site.
|
|
||
Click the individual point group symbols to go to the '3D Structures' section visualizing several examples of molecules belonging to the
corresponding groups. An overview on various structures of the different point groups is also provided in the
'Symmetry of Molecules - Examples' section.
 |
|||
As was outlined in the previous section, all molecules (or molecular conformations) belonging to the C1, Cn,
Dn, or the (very rare) T, O, or I point groups MUST be chiral. All other point groups include symmetry elements that
(any Sn axis, including mirror planes σ = S1 or inversion centers i = S2)
require a molecular geometry to be superimposable to its mirror image geometry, and therefore these molecules MUST be achiral
(see also the section 'Molecular Structures of Organic Compounds - Chirality'). On the other hand, polar molecules must have a permanent electric dipole moment. Only molecules belonging to the point groups Cn (including C1), Cnv, and Cs, may have a permanent dipole moment, which in the case of Cn and Cnv must lie along the rotation axis. All other point groups posses symmetry operations that include Cn axes (n > 1, and thus no dipole moment perpendicular to these axes), and symmetry operations which take one end of the molecule into the other (therefore no dipole moment along the principal axis can be observed).
|
|||