| TUD Organische Chemie | Immel | Graphics | MolArch+ | Animations | Conformation of Sucrose | View or Print (this frame only) |
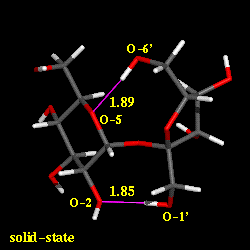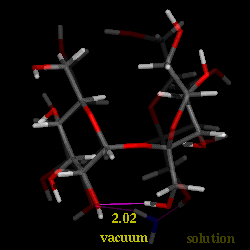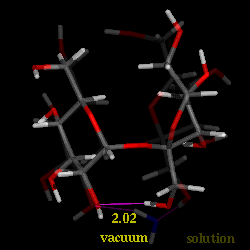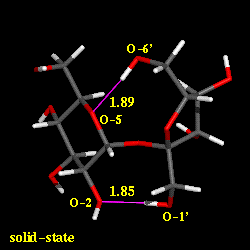
Conformational analysis of sucrose was carried out using the PIMM force-field[1], revealing three different energy-minimum geometries for the isolated molecule under in vaccum conditions. The conformations differ in their intersaccharidic torsion angles and intramolecular hydrogen bonding patterns; the global energy-minimum geometry closely resembles the solid-state conformation. The solution conformation of sucrose in water was investigated using molecular dynamics with explicit incorporation of water (sucrose in a truncated octahedron periodic box with 571 water molecules) and umbrella sampling techniques (GROMOS force-field[2]). Based on these molecular modelings, we have found with high signifigance a water molecule bridging the two sugar moieties through hydrogen bonding interactions. The animation displays a 'morphed' sequence of the solid-sate geometry, the global energy-minimum vaccum-structure, and the solution conformation of sucrose (capped-stick models, respectively). The characteristic hydrogen bonds are indicated by pink lines, the distances are shown in Angstroems.
 |
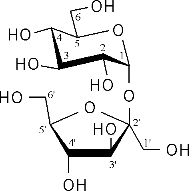 sucrose |
References
[1] |
(a) H. J. Lindner, PIMM88 - Closed Shell PI-SCF-LCAO-MO- Molecular Mechanics Program, Technical University of Darmstadt, 1988. - (b) H. J. Lindner, Tetrahedron 1974, 30, 1127-1132. - (c) A. E. Smith, Ph.D. Thesis, Technical University of Darmstadt, 1989. - (d) A. E. Smith and H. J. Lindner, J. Comput.-Aided Mol. Des. 1991, 5, 235-262. |
[2] |
(a) W. F. van Gunsteren and H. J. C. Berendsen, Groningen Molecular Simulation (GROMOS) Library Manual, Biomos, Nijenborgh 16, Groningen, The Netherlands, 1987. - (b) W. F. van Gunsteren and H. J. C. Berendsen, Angew. Chem. 1990, 102, 1020-1055; Angew. Chem. Int. Ed. Engl. 1990, 29, 992-1023. |