| TUD Organische Chemie | Immel | Publications | Lectures | Abstract 05 | View or Print (this frame only) |
Stefan Immel
3rd Domestic Meeting on "Bioactive Carbohydrates", hosted by Prof. Dr. N. Kashimura and Prof. Dr. S. Kitamura at Kyoto Prefectural University, Japan, November 7, 1998, Abstract 3-10.
Molecular dynamics simulations of α-cyclodextrin (1) in water were used to analyze hydrogen-bonding, hydration properties, and water exchange phenomena in detail (Figs. 1 and 2). Of the approx. 105-115 water molecules contained in the first hydration sphere of α-CD, an average of 34.2+-2.5 are engaged into 37.3+-2.8 hydrogen bonding interactions with the solute; the cavity itself is filled with an average of 6.2 H2O molecules.
 |
 |
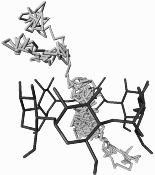 |
| Fig. 1: MD-derived relative water densities around α-CD in solution: dark shading indicates enhanced water probabilities, whereas light areas correspond to low or bulk phase water densities (left: front view perpendicular to the CD macrocycle, right: side view). | Fig. 2: 120 ps partial MD trajectory of the diffusion of a single water molecule through the CD cavity. | |
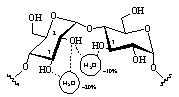
All hydroxyl groups are engaged in extensive H-bonding interactions with the surrounding solvent. Preferred are interresidue interactions of the 2-O - O-3' type between adjacent glucose units, but water-mediated hydrogen bond bridges (i.e. water molecules H-bonded to two hydroxyl groups simultaneously, cf. Fig. 3) were also found to persist with high significance.
| Fig. 3: In α-CD interresidue hydrogen bonds of the 2-O - O-3' type occur with high probability and long life-spans of around 30 ps, whereas the intra-residue interaction is disfavored. Water-mediated H-bond bridges are observed with probabilities of around 10-20 %. | 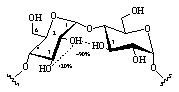 |
 |
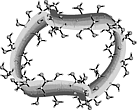
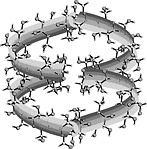
The hydrophobic characteristics of small- and large-ring CDs (cf. ribbon models shown below) were studied by molecular modeling and color-coded projection of the molecular lipophilicity patterns onto the solvent-accessible surfaces of the cyclodextrins[1].
 |
 |
 |
 |
|
 |
 |
 |
 |
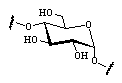
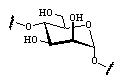
Out of the large number of cyclooligosaccharides consisting of other sugar units than glucose, the cyclomannins, cycloallins, and in particular the cycloaltrins appear most interesting. In α-cycloaltrin (α-CA 2, i.e. cyclo[D-Altp α(1→4)]6) inversion of the configuration at C-2 and C-3 is likely not only to introduce additional strain into the macrocycle, but it also renders the monosaccharide units with considerable flexibility.
α-Cycloaltrin (2)
adopts in the solid-state[2] a unique C3 symmetrical
conformation with nearly perfect
4C1 and
1C4
chairs in an alternating sequence (2a).
1H and 13C NMR
data in D2O at 30°C and 4°C
indicate a dynamic conformational equilibrium of at least two different altrose
geometries with almost equal energies in the macrocycle.
Unlike the conformation 2a (Fig. 4) which features side-on indentations only,
the intermediate all-OS2
structure 2b in the
4C1![]() OS2
OS2![]() 1C4
equilibrium features a "through-going" central cavity (Fig. 4). We have
investigated the dynamic processes in 2 by means of molecular dynamics (MD)
simulations in aqueous solution.
1C4
equilibrium features a "through-going" central cavity (Fig. 4). We have
investigated the dynamic processes in 2 by means of molecular dynamics (MD)
simulations in aqueous solution.
| 2a |  |
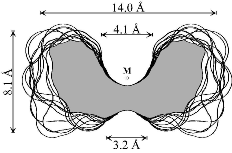 |
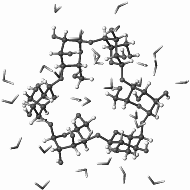 |
| 2b |  |
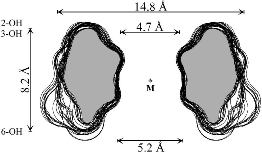 |
 |
| [1] |
On the hydrophobic characteristics of cyclodextrins: computer-aided visualization of molecular lipophilicity patterns. F. W. Lichtenthaler and S. Immel, Liebigs Ann. Chem. 1996, 27-37. |
| [2] |
Synthesis, structure, and conformational features of α-cycloaltrin: a cyclooligosaccharide with alternating 4C1/1C4pyranoid chairs. Y. Nogami, K. Nasu, T. Koga, K. Ohta, K. Fujita, S. Immel, H. J. Lindner, G. E. Schmitt, and F. W. Lichtenthaler, Angew. Chem. Int. Ed. Engl. 1997, 36, 1899-1902. |
Additional Graphics: Cyclodextrins