| TUD Organische Chemie | Immel | Publications | Papers | Abstract 24 | View or Print (this frame only) |
Holger Gohlke, Stefan Immel, and Frieder W. Lichtenthaler
Carbohydr. Res., 1999, 321, 96-104.
The conformational features of small cyclogalactins composed of β(1→3)- and β(1→6)-linked galactofuranose units, i.e. cyclo[D-Galf β(1→3)]n with n = 4 (1) and 5 (2), and cyclo[D-Galf β(1→6)]n with n = 3 (3) and 4 (4), were investigated by means of Monte-Carlo simulations. The flexibility of the macrocyclic backbone strongly favors bent and asymmetrical conformations over round geometries. Generation of the molecular surfaces of the global energy-minimum structures reveal disk-type shapes for 1 - 4 without through-going central cavities, yet distinct indentations close to the O-2 / O-3-groups, respectively. The molecular lipophilicity patterns prove these surface dents to be hydrophobic for the β(1→6)-linked cyclogalactins 3 and 4, whereas their β(1→3)-linked counterparts display an inverse situation with a hydrophobic outer core structure.
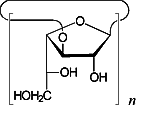 |
cyclo[D-Galf β(1→3)]n n = 4,5 |
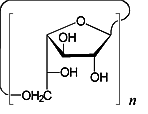 |
cyclo[D-Galf β(1→6)]n n = 3,4 |
![cyclo[D-Gal f beta(1->3)]4](graphics03.gif) |
![cyclo[D-Gal f beta(1->3)]5](graphics04.gif) |
![cyclo[D-Gal f beta(1->6)]3](graphics05.gif) |
![cyclo[D-Gal f beta(1->6)]4](graphics06.gif) |
| cyclo[D-Galf β(1→3)]4 1 |
cyclo[D-Galf β(1→3)]5 2 |
cyclo[D-Galf β(1→6)]3 3 |
cyclo[D-Galf β(1→6)]4 4 |
![surface of cyclo[D-Gal f beta(1->3)]4](graphics07.jpg) |
![surface of cyclo[D-Gal f beta(1->3)]5](graphics08.jpg) |
![surface of cyclo[D-Gal f beta(1->6)]3](graphics09.jpg) |
![surface of cyclo[D-Gal f beta(1->6)]4](graphics10.jpg) |
![cross-cut of cyclo[D-Gal f beta(1->3)]4](graphics11.jpg) |
![cross-cut of cyclo[D-Gal f beta(1->3)]5](graphics12.jpg) |
![cross-cut of cyclo[D-Gal f beta(1->6)]3](graphics13.jpg) |
![cross-cut of cyclo[D-Gal f beta(1->6)]4](graphics14.jpg) |
| Figure 1: Chemical formulas (top row) and global energy-minimum structures of the cyclogalactofuranosides 1 - 4 with their contact surfaces in dotted form (center). All structures are displayed perpendicular to the macrocycles with the O-2 / O-3 sides facing the viewer. In addition, surface cross-section plots perpendicular to the mean macro-ring planes are shown (bottom row), with approximate molecular dimensions in Å. | |||
 |
|
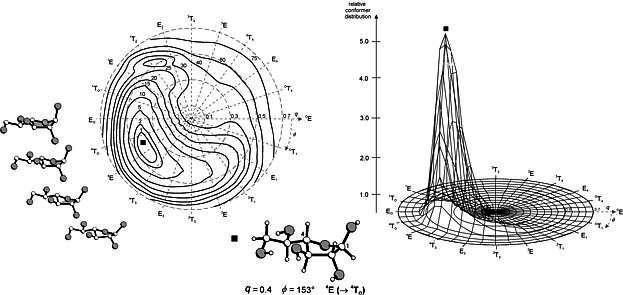
Figure 2:
Left: Adiabatic energy map of β-D-galactofuranose as a function of the furanose Cremer-Pople parameters
- i.e. the puckering amplitude q and the phase angle
f - calculated by molecular
mechanics using the PIMM91 force-field (energies are given in kJ/mol).
The bold-faced square marks the global energy minimum corresponding
to the conformation displayed underneath (4E form slightly
distorted towards the 4TO geometry); the
structures to the left represent the furanose conformations within
4T3 |
 |
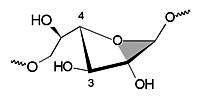 |
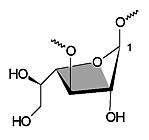
| 1E | 4T3 |
| Figure 3: Schematic representation of the furanose geometries calculated for cyclogalactofuranosides 1 - 4: 1E conformation for the β(1→3)-linked cyclooligosaccharides 1 and 2 (left) versus the 4T3 form for the β(1→6)-analogs 3 and 4 (right). | |
Additional Graphics: Cyclogalactins