| TUD Organische Chemie | Immel | Publications | Papers | Abstract 26 | View or Print (this frame only) |
S. Immel
Proceedings of the 10th Internat. Symp. on Cyclodextrins (Ed.: J. Szejtli), Mia Digital Publ., Ann Arbor, Michigan, 2000, pp. 24-31.
In contrast to the static "lock-and-key" type complexation behavior of the cyclodextrins, cyclooligosaccharides in which one or all glucose units are replaced against
α-D-altrose residues are thoroughly flexible, and the pyranose units can adopt various conformations along the
4C1 ![]() OS2 / 3,OB
OS2 / 3,OB ![]() 1C4
pathway (Fig. 1). Thus, α-cycloaltrin (α-CA, 1)[1] and mono-altro-β-CD (2)[2]
are the first prominent examples of cyclooligosaccharide hosts able to realistically mimic an "induced-fit" type mechanism of inclusion complex formation.
1C4
pathway (Fig. 1). Thus, α-cycloaltrin (α-CA, 1)[1] and mono-altro-β-CD (2)[2]
are the first prominent examples of cyclooligosaccharide hosts able to realistically mimic an "induced-fit" type mechanism of inclusion complex formation.
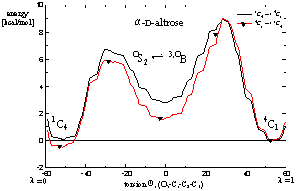 |
Fig. 1. Calculated free energy profile ("potential of mean force", CHARMM) of α-D-altrose in aqueous solution as a function of the pyranose ring conformation; energies are given in kcal/mol. Both the 1C4 (left side of the plot) and 4C1 (right side) ring geometries have almost equal energies, whilst the intermediate OS2 / 3,OB forms are slightly less stable. | |
|
||
 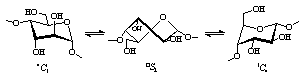 |
 |
|
|
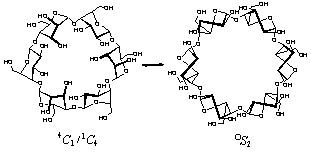
Fig. 2. α-CA (1) adopts a multitude of conformations along the
4C1 |
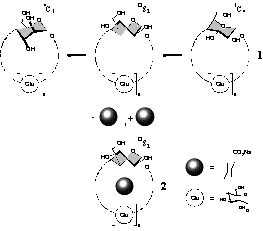
Fig. 3. Induced-fit type complexation of mono-altro-β-cyclodextrin (2) with adamantane-1-carboxylate. |