| TUD Organische Chemie | Immel | Publications | Poster | Abstract 05 | View or Print (this frame only) |
Stefan Immel and Frieder W. Lichtenthaler
8th European Carbohydrate Symposium (EUROCARB VIII), Seville, Spain, July 2-7, 1995, Abstract B38.
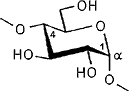
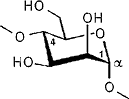
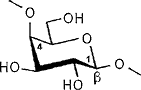
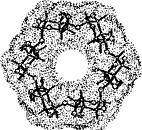
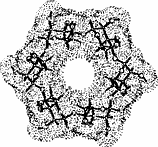
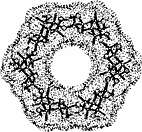
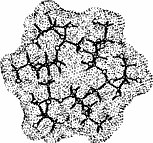
The color-coded projections of molecular lipophilicity patterns (MLP) on molecular surfaces provide a new look at the hydrophobic topographies of cyclodextrins. By comparing the starch-derived cyclodextrins containing 6 (α-CD, 1) to 9 glucose units[1] with their non-natural small-ring counterparts with 3, 4, or 5 glucose residues, it becomes obvious that the cycloglucopentaoside is still capable of forming inclusion complexes, whilst the smaller homologs are amenable to hydrophobic binding via a surface indentation only[2]. Isomeric CD's, such as the α(1→4) linked cyclomannins (e.g. 2) exhibit narrowed, but pronouncedly hydrophobic cavities[3]. For the β(1→4) linked cyclogalactins ("inside-out" CD's) an inverse situation prevails, the central cavities in the α-cyclogalactin (3)being more hydrophilic than the outer surface areas[3].
 |
 |
 |
 |
 |
 |
 |
 |
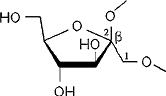
Distinct differences in their MLPs and the absence of a central cavity
reveal that the inulin-derived α-cyclofructin
4 is devoid of similar inclusion phenomena[4].
The visualization of the hydrophobic nature of the central channel
of Vh-amylose sheds
further light on the complex formation properties of carbohydrate
macromolecules[5].
_______________
[1] F. W. Lichtenthaler, S. Immel, Liebigs Ann. Chem. 1996, 27-37.
[2] S. Immel, J. Brickmann, F. W. Lichtenthaler, Liebigs Ann. Chem. 1995, 929-940.
[3] F. W. Lichtenthaler, S. Immel, Tetrahedron: Asymmetry 1994, 5, 2045-2060.
[4] S. Immel, F. W. Lichtenthaler, Liebigs Ann. Chem. 1996, 39-44.
[5] F. W. Lichtenthaler, S. Immel, Int. Sugar J. 1995, 97, 12-22.
Additional Graphics: Cyclodextrin Isomers