| TUD Organische Chemie | Immel | Publications | Poster | Abstract 06 | View or Print (this frame only) |
Stefan Immel, Guido Schmitt, and Frieder W. Lichtenthaler
XVIIIth International Carbohydrate Symposium, Milano, Italy, July 21-26, 1996, Abstract AO027.
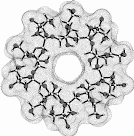
Cyclofructins composed of six (α-CF, 1) to ten (5) β(1→2)-linked fructofuranose units (i.e. cyclo[D-Frufβ(1→2)]n with n = 6 - 10) were subjected to conformational analysis using "Monte-Carlo" simulations based on the PIMM91[1] force field[2,3]. Far-reaching similarities and identical over-all conformations of the solid-state geometry[4] of -cyclofructin (1) and its computer-generated form provide information about the reliability of the computational analysis.
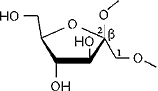 |
1 cyclo[D-Frufβ(1→2)]6
2 cyclo[D-Frufβ(1→2)]7 3 cyclo[D-Frufβ(1→2)]8 4 cyclo[D-Frufβ(1→2)]9 5 cyclo[D-Frufβ(1→2)]10 |
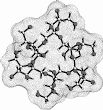
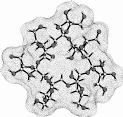
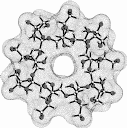
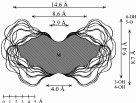
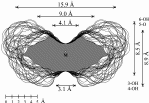
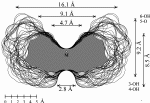
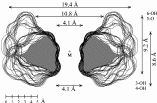
Calculation of the molecular surfaces for the energy-minimum structures establishes a disk-type shape of the cyclofructins with six to eight residues, ring enlargement to nine and ten residues leads to torus-shaped molecules with central cavities that conceivably allow for the formation of inclusion complexes. The color-coded projection of molecular lipophilicity patterns (MLPs)[5] and electrostatic potential profiles (MEPs) onto these surfaces displays the crown ether-like properties of the disk-shaped cyclofructins. The central cavities of 4 and 5 should be amenable to the formation of inclusion complexes similar to those formed by cyclodextrins.
 |
 |
 |
 |
 |
 |
 |
 |
 |
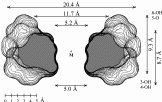 |
Fig. 1. Geometries with the dotted contact surfaces and cross section plots of the global energy-minimum structures (PIMM91) of the cyclofructins 1 - 5 composed of six to ten (1→2)-linked fructose units.
| [1] |
A. E. Smith, H. J. Lindner,J. Comput.-Aided Mol. Des. 1991, 5, 235-262. |
| [2] |
S. Immel, F. W. Lichtenthaler, Liebigs Ann. Chem. 1996, 39-44. |
| [3] |
S. Immel, F. W. Lichtenthaler, to be submitted. |
| [4] |
M. Sawada, T. Tanaka, Y. Takai, T. Hanafusa, T. Taniguchi, M. Kawamura, T. Uchiyama, Carbohydr. Res. 1991, 217, 7-17. |
| [5] |
M. Waldherr-Teschner, T. Goetze, W. Heiden, M. Knoblauch, H. Vollhardt, J. Brickmann, in: Advances in Scientific Visualization (Eds.: F. H. Post, A. J. S. Hin), Springer Verlag, Heidelberg, 1992, pp. 58-67. |
Additional Graphics: Cyclofructins