| TUD Organische Chemie | Immel | Publications | Poster | Abstract 07 | View or Print (this frame only) |
Frieder W. Lichtenthaler, Hans J. Lindner, Stefan Immel, and Guido E. Schmitt
9th European Carbohydrate Symposium (EUROCARB XI), Utrecht, Netherlands, July 6-11, 1997, Abstract C5.
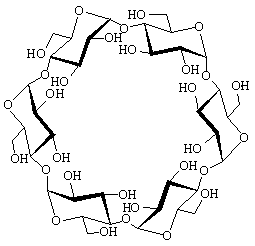
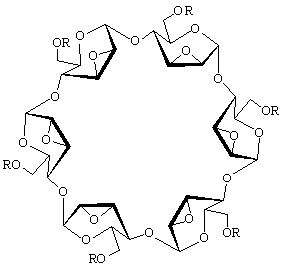
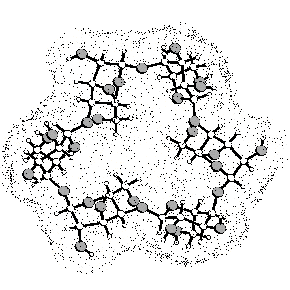
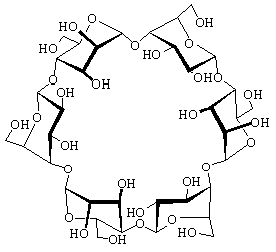
The structural characteristics of α-cycloaltrin (3), readily
available from α-cyclodextrin (1) by a straightforward four-step
protocol[1] with 2,3-anhydro-α-cyclomannin (2)
as the key intermediate, has been unraveled using X-ray techniques,
800 MHz spectra (D2O at 30 and 4°C) and molecular
modeling (MD in water). In the solid-state, the altropyranoid
rings adopt nearly perfect 4C1 and 1C4
chairs in an alternating sequence, entailing the macrocycle to
be devoid of a through going cavity. From HTA calculations i.e.
toward vacuum boundary conditions, the allskew (twistboat)
0S2 geometry emerges as the global energy
minimum structure. In water, the altropyranoid rings in 3
adopt various conformations within the 1C4
![]() 3H2
3H2
![]() 0S2 range.
0S2 range.
 |
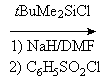 |
 |
|
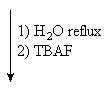 | ||
 |
 | |
[1] Y. Nogami, K. Fujita, K. Ohta, K. Nasu, H. Shimada, C. Shinohara, and T. Koga, (J. Szejtli, L. Szente, Eds.), Proceedings of the 8th Internat. Symp. on Cyclodextrins, Kluwer Acad. Publ., Dordrecht, 1996, pp. 99-102.
Additional Graphics: Cycloaltrins