| TUD Organische Chemie | Immel | Publications | Poster | Abstract 08 | View or Print (this frame only) |
Stefan Immel, Guido Schmitt, and Frieder W. Lichtenthaler
9thInternational Symposium on Cyclodextrins, Santiago de Compostela, Spain, May 31-June 3, 1998, Abstract 2-O-5.
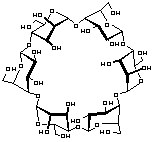
α-Cycloaltrin, readily available from -cyclodextrin via a straightforward four-step protocol[1], adopts in the solid-state[2] a C3 symmetrical conformation with nearly perfect 4C1 and 1C4 chairs in an alternating sequence, but lacks a "through-going" cavity.
 |
 |
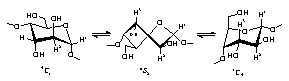
1H and
13C NMR experiments of
α-cycloaltrin show a complex conformational
equilibrium
4C1 |
 |
 |
 |
Molecular dynamics (MD) simulations and temperature-dependent 800 MHz
1H and
13C NMR spectra (D2O
at 30 and 4°C) point toward a fast dynamic equilibrium with
preferred altrose conformations in the
1C4![]() OS2 range.
Low-temperature broadening of the C-4 and C-5 NMR signals indicate multiple conformations
of α-CA in solution which are likely to ellaborate central
cavities similar to those formed by cyclodextrins.
OS2 range.
Low-temperature broadening of the C-4 and C-5 NMR signals indicate multiple conformations
of α-CA in solution which are likely to ellaborate central
cavities similar to those formed by cyclodextrins.
| [1] |
Y. Nogami, K. Fujita, K. Ohta, K. Nasu, H. Shimada, C. Shinohara, and T. Koga, J. Inclusion Phenom. Mol. Recognit. Chem. 1996, 25, 57-60. |
| [2] |
Y. Nogami, K. Nasu, T. Koga, K. Ohta, K. Fujita, S. Immel, H. J. Lindner, G. E. Schmitt, and F. W. Lichtenthaler, Angew. Chem. 1997, 109, 1987-1991; Angew. Chem. Int. Ed. Engl. 1997, 35, 1899-1902. |
Additional Graphics: Cycloaltrins