| TUD Organische Chemie | Immel | Publications | Poster | Abstract 09 | View or Print (this frame only) |
Stefan Immel, Guido Schmitt, and Frieder W. Lichtenthaler
9thInternational Symposium on Cyclodextrins, Santiago de Compostela, Spain, May 31-June 3, 1998, Abstract 2-P-3.
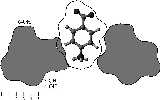
Conformational analysis of cyclofructins[1] composed of six (α-CF, 1) to ten (5) β(1→2)-linked fructofuranose units (i.e. cyclo[D-Frufβ(1→2)]n with n = 6 - 10) was carried out in vacuum and aqueous solution using molecular mechanics (Monte Carlo) and dynamics simulation techniques.
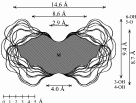
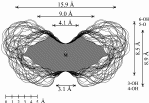
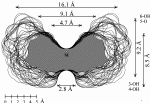
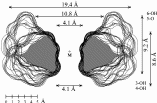
| Calculation of molecular surfaces and cross section cuts (cf. below) for the energy-minimum geometries reveals a disk-type shape of 1 - 3 without a "through-going" cavity. The larger ring homologs with nine (4) and ten (5) fructose units feature torus-like structures with central cavities of 4.1 or 5.2 Å diameter, respectively. Evaluation of the molecular electrostatic and lipophilicity patterns displays the crown-ether like properties of the cyclofructins 1 - 5. The pronouncedly hydrophobic cavities for 4 and 5 should be amenable to the formation of inclusion complexes similar to those formed by cyclodextrins. |
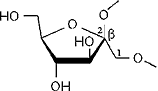 cyclo[D-Fruf β(1→2)]n with n = 6 - 10 |
 |
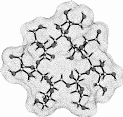 |
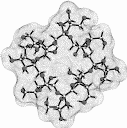 |
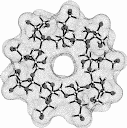 |
 |
 |
 |
 |
 |
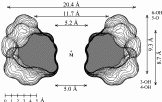 |
| Cross-section contours through the surfaces of the most stable structures computed for the inclusion complexes of 4 with β-alanine (left) and 5 with p-amino benzoic acid (right). |  |
 |
| [1] |
S. Immel, F. W. Lichtenthaler, Liebigs Ann. Chem. 1996, 39-44. |
Additional Graphics: Cyclofructins