| TUD Organische Chemie | Immel | Publications | Poster | Abstract 10 | View or Print (this frame only) |
Holger Gohlke, Stefan Immel, Frieder W. Lichtenthaler, and Guido Schmitt
9thInternational Symposium on Cyclodextrins, Santiago de Compostela, Spain, May 31-June 3, 1998, Abstract 2-P-4.
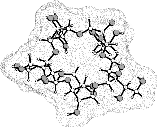
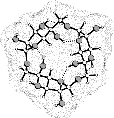
Various cyclooligosaccharides composed of β(1→3)-, β(1→5)-, and β(1→6)-linked galactofuranose units have been prepared by cycloglycosylation of tritylated 1,2-O-(1-cyano)ethylidene-derivatives of D-galactofuranose[1], yet information as to their molecular geometries and particularly to their inclusion complex behavior is essentially non-existent. To get a first assessment of the conformational features involved we have subjected the cyclogalactins 1 - 4 to PIMM91 Monte Carlo simulations:
 |
cyclo[D-Galfβ(1→3)]4 1 cyclo[D-Galfβ(1→3)]5 2 |
 |
cyclo[D-Galfβ(1→6)]3 3 cyclo[D-Galfβ(1→6)]4 4 |
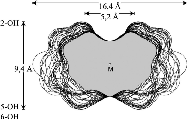
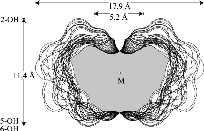
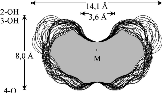
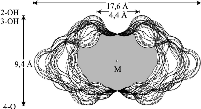
The results reveal a comparatively high flexibility of the cyclic furanosides, rendering the molecules with irregular, asymmetric overall shapes rather than symmetrical geometries. In contrast to cyclogalactins composed of pyranoid units[2], calculation of the corresponding molecular surfaces and the cross section cuts of 1 - 4 displays them to be disk-shaped and devoid of central cavities.
 |
 |
 |
 |
 |
 |
 |
 |
| [1] |
N. K. Kochetkov, S. A. Nepogodiev, L. V. Backinowsky, Carbohydr. Res. 1989, 185, C1-C3; Tetrahedron 1990, 46, 139-150. |
| [2] |
F. W. Lichtenthaler, S. Immel, Tetrahedron: Asymmetry 1994, 5, 2045-2060. |
Additional Graphics: Cyclogalactins