| TUD Organische Chemie | Immel | Publications | Poster | Abstract 11 | View or Print (this frame only) |
Stefan Immel, Guido Schmitt, and Frieder W. Lichtenthaler
XIXth International Carbohydrate Symposium, San Diego, California, August 9-14, 1998, Abstract AP124.
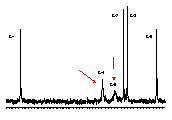
α-Cycloaltrin (1)[1] adopts in the solid-state[2] a unique C3 symmetrical conformation with nearly perfect 4C1 and 1C4 chairs in an alternating sequence (1a). 1H and 13C NMR data in D2O at 30°C and 4°C (cf. Fig. 1) indicate a dynamic conformational equilibrium of at least two different altrose geometries with almost equal energies in the macrocycle.
| Fig. 1: 13C NMR (75 MHz) spectra of (α-CA (1) in D2O at 30°C (left) and 4°C (right) indicate a pronounced low-temperature broadening of the C-4 and C-5 signals, and hence, a dynamic equilibrium between at least two different altrose conformations. | 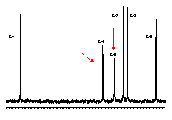 |
 |
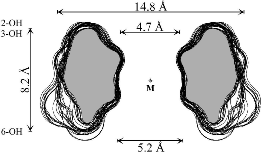
Unlike the conformation 1a (Fig. 2) which features side-on indentations only,
the intermediate all-OS2
structure 1b in the
4C1![]() OS2
OS2![]() 1C4
equilibrium features a "through-going" central cavity (Fig. 2). We have
investigated the dynamic processes in 1 by means of molecular dynamics (MD)
simulations in aqueous solution, focusing in particular on the hydration
properties and water exchange phenomena of 1a and 1b, defining the first
hydration shells of both geometries (Fig. 2).
1C4
equilibrium features a "through-going" central cavity (Fig. 2). We have
investigated the dynamic processes in 1 by means of molecular dynamics (MD)
simulations in aqueous solution, focusing in particular on the hydration
properties and water exchange phenomena of 1a and 1b, defining the first
hydration shells of both geometries (Fig. 2).
| 1a | 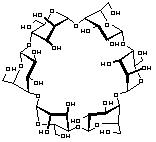 |
 |
 |
| 1b | 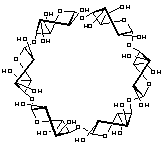 |
 |
 |
| [1] |
Y. Nogami, K. Fujita, K. Ohta, K. Nasu, H. Shimada, C. Shinohara, and T. Koga, J. Inclusion Phenom. Mol. Recognit. Chem. 1996, 25, 57-60. |
| [2] |
Y. Nogami, K. Nasu, T. Koga, K. Ohta, K. Fujita, S. Immel, H. J. Lindner, G. E. Schmitt, and F. W. Lichtenthaler, Angew. Chem. 1997, 109, 1987-1991; Angew. Chem. Int. Ed. Engl. 1997, 35, 1899-1902. |
Additional Graphics: Cycloaltrins