| TUD Organische Chemie | Immel | Publications | Poster | Abstract 12 | View or Print (this frame only) |
Stefan Immel and Guido Schmitt
XIXth International Carbohydrate Symposium, San Diego, California, August 9-14, 1998, Abstract AO008.
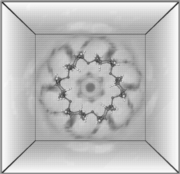
Molecular dynamics simulations of α-cyclodextrin (1) with explicit consideration of the solvent (609 water molecules, CHARMM force-field, periodic boundary conditions of the truncated octahedron, t = 600 ps, T = 300 K, p = 1 bar) were used to analyze hydrogen-bonding, hydration properties, and water exchange phenomena in detail. Of the approx. 105-115 water molecules contained in the first hydration sphere of α-CD, an average of 34.2+-2.5 are engaged into 37.3+-2.8 hydrogen bonding interactions with the solute. The computed relative 3D-anisotropic water densities (Fig. 1) clearly indicate the first hydration sphere, with water molecules trapped in relatively well defined positions within the cavity. The cavity itself is filled with an average of 6.2 H2O molecules with correlation times (i.e. average "residence" times in the cavity) of 11.5 ps. Monitoring the diffusion of individual water molecules into as well as through the cavity (Fig. 2) yielded an approx. ratio of 1.15 : 1 for the probability of water molecules entering from the 2/3-OH or the opposite 6-CH2OH side.
 |
 |
 |
| Fig. 1: MD-derived relative water densities around α-CD in solution: dark shading indicates enhanced water probabilities, whereas light areas correspond to low or bulk phase water densities (left: front view perpendicular to the CD macrocycle, right: side view). | Fig. 2: 120 ps partial MD trajectory of the diffusion of a single water molecule through the CD cavity. | |
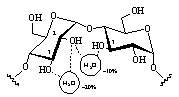
All hydroxyl groups are engaged in extensive H-bonding interactions with the surrounding solvent. Due to their location close to the hydrophobic 6-CH2OH side [1] and the central cavity of the CD torus, the oxygens O-1 and O-5 are least effected by solvation. Preferred are interresidue interactions of the 2-O - O-3' type between adjacent glucose units, but water-mediated hydrogen bond bridges (i.e. water molecules H-bonded to two hydroxyl groups simultaneously, cf. Fig. 3) were also found to persist with high significance.
| Fig. 3: In α-CD interresidue hydrogen bonds of the 2-O - O-3' type occur with high probability and long life-spans of around 30 ps, whereas the intra-residue interaction is disfavored. Water-mediated H-bond bridges are observed with probabilities of around 10-20 %. |  |
 |
| [1] |
F. W. Lichtenthaler and S. Immel, Liebigs Ann. Chem. 1996, 27-37. |
Additional Graphics: Cyclodextrins