| TUD Organische Chemie | Immel | Publications | Poster | Abstract 13 | View or Print (this frame only) |
Toshio Nakagawa, Stefan Immel, Hans Jörg Lindner, and F. W. Lichtenthaler
XVIIth Japanese Cyclodextrin Symposium, Osaka, Japan, October 13-14, 1999.
Periodate oxidation of α- to γ-cyclodextrins followed by borohydride reduction gave the 30-, 35-, 40-membered cyclic crown acetals, which were isolated and characterized in the form of their achiral crystalline per-O-acetates. An X-ray analysis of the α-CD derived crown acetal revealed a unique topology: three acetoxyethylidene units are located inside the macro-ring, and the other three outside; of the six diacetoxy-meso-butane units, three are in a gauche and the other three in a trans arrangement.
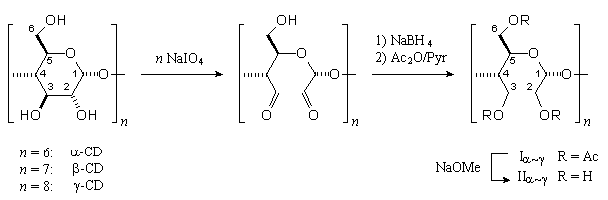
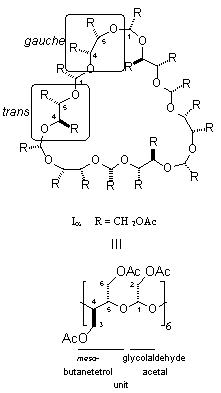 |
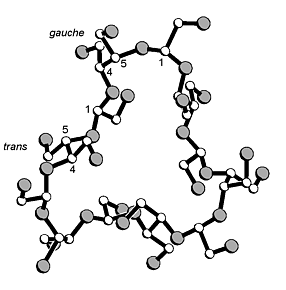 |
| [1] |
L. Kandra, A. Liptak, A. I. Jodal, P. Nanasi, J. Szejtli, J. Inclusion Phenom. 1984, 2, 869-875. |
Additional Graphics: Crown Acetals