| TUD Organische Chemie | Immel | Publications | Related | Abstract 03 | View or Print (this frame only) |
Frieder W. Lichtenthaler and Stefan Mondel
Carbohydr. Res. 1997, 303, 293-302.
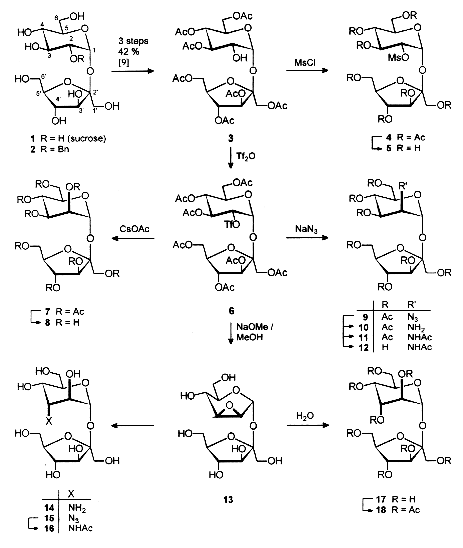
Preparatively useful procedures were developed for the conversion of sucrose into isomers with manno- and altro-configuration in the pyranoid part. Key compound proved to be the 2g-triflate peracetate smoothly generated from the readily accessible 2g-OH-free heptaacetyl-sucrose by standard triflation. SN2-displacement of the triflate by acetate (manno-sucrose) or azide (→manno-sucrosamine) proceeded in high yields, exposure to deacetylation conditions effected the displacement by the 3g-OH thus liberated, resulted in the manno-sucrose-2,3-epoxide; oxirane ring opening with water, ammonia, or azide proceede in diaxial fashion to provide altro-sucrose, and its 3-amino and 3-azido derivatives, respectively. Sweetness evaluations proved manno-sucrose to be about 5 times less sweet than than sucrose, whereas the altro-isomer is devoid of sweetness, correlating well with our refined AH-B-X structure-sweetnes concept.