| TUD Organische Chemie | Immel | Publications | Lectures | Abstract 01 | View or Print (this frame only) |
Stefan Immel and Frieder W. Lichtenthaler
7th European Carbohydrate Symposium (EUROCARB VII), Cracow, August 22-27, 1993, Abstract C026.
Computer-aided modeling studies of the conformations, the molecular electrostatic potential (MEP), and the lipophilicity potential (MLP) profiles of sucrose, fructose, and non-carbohydrate sweeteners reveal close similarities in their hydrophobic and hydrophilic surface regions on opposite molecular sides, the former being a crucial factor in guiding the substrate into the sweet taste receptor and locking it into the correct position for eliciting the sweetness response. The hydrophilic region contains the AH-B couple of functional groups amenable to hydrogen bonding with a complementary receptor-based AH-B unit.
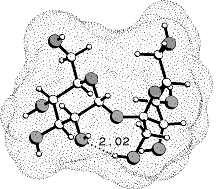
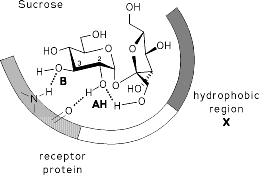
The AH-B-assignments made are supported by the MEP's shown, leading to new
insights into the physico-chemical properties of the glucophoric unit of
sweeteners. Further corroboration of the new concept is provided by the
sweetness data of a variety of analogs of these compounds.
Additional Graphics: Sucrose / High-Potency Sweeteners