| TUD Organische Chemie | Immel | Publications | Lectures | Abstract 02 | View or Print (this frame only) |
Stefan Immel and Frieder W. Lichtenthaler
207th ACS Meeting, San Diego, March 13-18, 1994, Abstract CARB 11.
Computer-aided modeling studies of the conformations, the molecular electrostatic potential (MEP), and the lipophilicity potential (MLP) profiles of sucrose, fructose, and non-carbohydrate sweeteners reveal close similarities in their hydrophobic and hydrophilic surface regions on opposite molecular sides, the former being a crucial factor in guiding the substrate into the sweet taste receptor and locking it into the correct position for eliciting the sweetness response. The hydrophilic region contains the AH-B couple of functional groups amenable to hydrogen bonding with a complementary receptor-based AH-B unit.
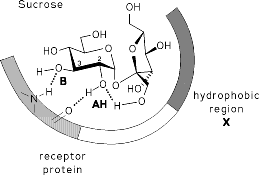
* F. W. Lichtenthaler, S. Immel, Sweet Taste Chemoreception, (G. G. Birch, M. A. Kanters, M. Mathlouthi, Eds.), Elsevier, Amsterdam, 1993, 21-61.
Additional Graphics: Sucrose / High-Potency Sweeteners