| TUD Organische Chemie | Immel | Publications | Poster | Abstract 04 | View or Print (this frame only) |
Stefan Immel and Frieder W. Lichtenthaler
XVIth International Carbohydrate Symposium, Paris, July 5-10, 1992, Abstract C163.
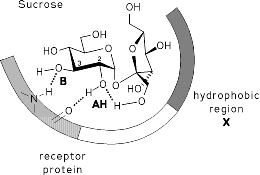
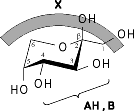
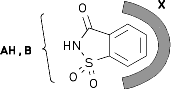
MOLCAD program-calculated MEP's (Molecular Electrostatic Potential) and MLP's (Molecular Lipophilicity Potential) on the contact surfaces of sucrose, sucralose, fructose, and some non-carbohydrate sweeteners (saccharin. cyclamate, and aspartame) provide new insights into structure-sweetness relationships and a reassignment of the AH-B-X glucophore. The hydrophobicity distributions show the lipophilic X-part to be an entire region rather than a specific corner of the, "sweetness triangle": in sucrose and sucralose encompassing the outside area of the fructofuranose moiety, in fructose the 1- and 6-CH2 groups, and in saccharin the aromatic ring system. The hydrophilic portions of all sweeteners are invariably located opposite to the hydrophobic region, and appear to contain the AH-B couple of the glucophore: the glucosyl-2- and 3-OH group in sucrose and sucralose, versus the 3,4-diol grouping in fructose, and the sulfamido-group in saccharin.
|
|
|
The results, particularly the MLP patterns presented, sustain the notion, that the hydrophobic regions are initiating the "docking procedure", guiding the hydrophilic AH-B site of the substrate into its proper position required, eliciting the sweet response via hydrogen bonding to a complementary receptor-based AH-B couple.
Additional Graphics: Sucrose / High-Potency Sweeteners