 MOLecular ARCHitecture+ - MolArch+ Movies MOLecular ARCHitecture+ - MolArch+ Movies
|
This page gives an overview on different types of molecular animations
that were generated using the MolArch +
program.
|
 |
 Ion Transport in Perowskit Ion Transport in Perowskit |
|
 SN2 Reaction SN2 Reaction |
The SN2 mechanism (bimolecular nucleophilic substitution) involves rear-side attack of a nucleophile at a
carbon atom, opposite to the leaving group being displaced. This one-step reactions proceed with inversion of
stereocenters through a trigonal-bipyramidal penta-coordinated carbon atom. The animations show the
degenerate SN2 reaction of (R)-2-bromo-butane with bromide yielding (S)-2-bromo-butane (racemization).
|
|
|
|
 Bromination of cis- and trans-2-Butene Bromination of cis- and trans-2-Butene |
Bromination of alkenes proceeds via the AdE2 mechanism (bimolecular electrophilic addition). First,
cyclic bromonium ions are formed, which undergo ring opening through rear-side attack by bromide. The over-all process
is a trans-addition of bromine. Starting from trans-2-butene, the
(2R,3S)- and (2S,3R)-2,3-dibromo-butanes (erythro pair) are formed through initial front- and rear-side attack of the
alkene, both compounds being identical, achiral meso-structures (homotopic carbon atoms
in the bromonium ion). Contrary, the bromination of cis-2-butene yields the enantiomers
(2R,3R)- and (2S,3S)-2,3-dibromo-butane (threo pair) as the racemate (enantiotopic carbon atoms
in the bromonium ion). The animations display the stereochemical course of all reactions leading to any
of the stereoisomeric products.
|
|
|
|
 Permanganate Oxidation of Methane Permanganate Oxidation of Methane |
These animations display the calculated mechanism of the C-H-oxidation of methane through permanganate (the surfaces shown in these examples are not molecular orbitals,
but simple test surfaces which I used for program development; if available, orbital data could have been used instead). Data provided
by T. Strassner, TU München, Germany.
|
|
|
|
 Lithiation of Triazine Lithiation of Triazine |
Lithiation of 5-methoxy- and 4-methoxy-1,2,3-triazine with lithium amide.
The various models displayed differ by the mode with which the lithium cation is complexed in the transition states.
Data provided by M. Urban, TU Darmstadt, Germany.
|
|
|
|
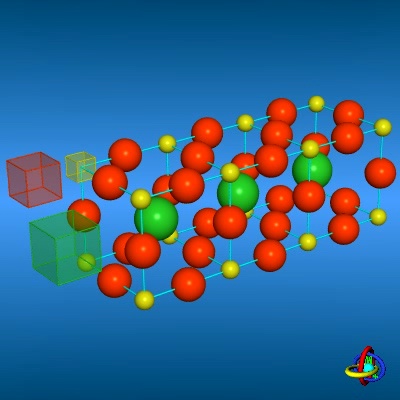
 Ion Transport Mechanism in Perowskit Type Lattices Ion Transport Mechanism in Perowskit Type Lattices |
The animations display a proposed mechanism for the ion transport in perowskit type lattices.
In this GaLaO3 structure, the Lanthanum (green spheres), Gallium (yellow) and Oxygen (orange-red) ions
migrate alternatingly from their original lattice positions to adjacent voids. In total, each cycle of four migration steps results
in a configuration in which the voids are displaced by just one unit-cell, and the corresponding ions have moved in the opposite direction.
Data provided by O. Schulz, TU Darmstadt, Germany.
|
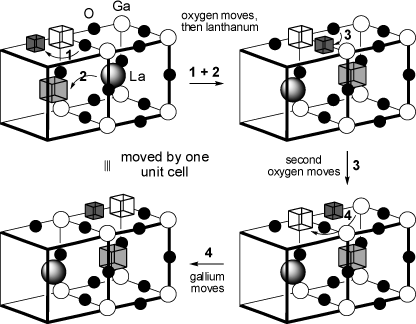
The above scheme shows only the relevant atomic positions (La: shaded spheres, Ga: white, Oygen: black), the boxes
correspond to lattice voids. Each cubic unit cell has dimensions 3.99 x 3.99 x 3.99 Å.
|
|
|
 Miscellaneous Animations Miscellaneous Animations |
The animations below are simple examples of spinning structures like the solid-state geometry of a linked
cyclodextrin derivative and a single molecule of pyrane.
In the center, the three-dimensional molecular electrostatic potenials
for benzene and salicylic-acid are displayed for their rotating structures.
The electrostatic potenials were rendered as transparent 3D contours with different color-scales (benzene: yellow for negative electrostatic potenials and
blue for positive potenials; salicylic-acid: red-green-blue color scale for negative to positive potentials).
|
The rightmost animations display 'endless flights' through the three-dimensional lattices of inorganic structures such as diamond, graphite, and a zeolite
(play these movies in loop-mode!).
|
|
|
|
|