| TUD Organische Chemie | Immel | Tutorials | Symmetry | Symmetry Elements and Operations | View or Print (this frame only) |
Symmetry elements and operations are prerequisites for the discussion of the symmetry of molecules.
This sections gives a short description of the basic terminology.
The symmetry of molecules is important for understanding the structures and properties of organic compounds. In chemistry, many phenomena may be easily explained by consideration of symmetry. The systematic discussion of symmetry is called group theory. the fundamental principles of symmetry analysis are explained in this section. Atoms or groups in molecules are called symmetry equivalent if they have the same atomic number or chemical constitution, and if they can be transposed into each other by the application of symmetry operations (see below). This equivalency plays an important role in the chemical properties of these atoms or groups, and in the process of stereo differentiation of these positions.
|
|
||
Symmetry operations are geometrical operations applied to molecules (or better, molecular models).
The application of a symmetry operation to a particular molecular geometry produces a spatial orientation which is indistinguishable from the initial orientation.
For example, rotating a water (H2O) molecule by 180° about an axis bisecting the H-O-H bond angle will produce a orientation of that molecule which
looks the same as its original orientation. In fact, this rotation leads to an exchange of both protons Ha and Hb, but as these two protons
may not be distinguished from each other, the resulting orientation of the water molecule is equivalent to the initial one. Another rotation of 180° about this axis
leads to an orientation which is identical to the starting geometry. The five basic symmetry operations that need to be considered are identity, rotation, reflection (mirroring), inversion, and rotary reflection. The symmetry properties of molecules are described as the set (combination) of valid symmetry operations for its molecular geometry. For each symmetry operation there is a corresponding symmetry element, with respect to which the symmetry operation is applied. For example, the rotation (a symmetry operation) is carried out around an axis which is the corresponding symmetry element. Other symmetry elements are planes (for reflections) and points (for inversions). All spatial points along these symmetry elements (i.e. the symmetry plane, axis, or center of inversion) do not change their positions during the corresponding symmetry operation. Common to all symmetry operations is that the geometrical center of a molecule does not change its position, all symmetry elements must intersect in this center. In fact, out of the five symmetry operations mentioned above only two (rotation and rotary reflection) are sufficient to describe all symmetry properties of molecules. Different (valid) combinations of symmetry operations that leave at least a single common point (the molecular center) unchanged give rise to the so called point groups (not all arbitrary combinations of symmetry operations and elements are possible, and some combinations of symmetry operations implicitly imply others, see below). When considering crystals, additional symmetries arising from translations through three-dimensional space need to be considered, giving rise to the more extensive space groups. The basic symmetry operations are: |
|||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
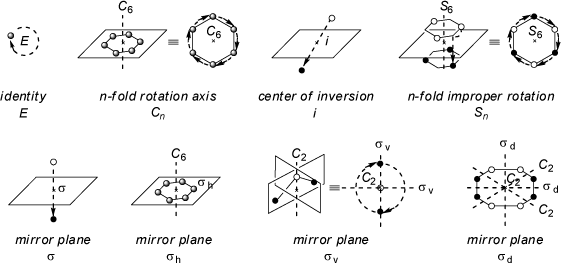
Examples for the different basic symmetry operations and symmetry elements are given in the scheme below:
 |
|||
Out of the five symmetry operations mentioned above only two (rotation and rotary reflection) are
sufficient to describe all symmetry properties of molecules. The identity operation is equivalent to a C1 axis of rotation, as rotation of
all objects around 360° about any axis produces the initial orientation only. On the other hand, a mirror plane is equivalent to a
S1 axis of rotary-reflection perpendicular to that plane. In addition, a center of inversion can be represented
by a S2 axis of improper rotation with any arbitrary orientation of this axis (all axes must intersect in the
inversion center, which also must be the center of geometry for any molecule).
 |
|||
Symmetry operations may not be combined arbitrarily with each other, but only in a limited number of variations which are the so called point groups.
Some symmetry operations implicitly imply others, as for example any S4 or C4 axis must be accompanied by a parallel
C2 axis. For example in benzene, the principal C6 axis simultaneously must be a C3 and a C2 axis
of symmetry, too. Similarly, any Sn axis with odd n is identical to a Cn axis in conjunction with a
horizontal mirror plane σh. However, certain combinations of some symmetry operations implicitly generate other symmetries. For example, the combination of any Cn axis with even n, together with a center of inversion i implicitly generates a horizontal mirror plane σh (which is perpendicular to the Cn axis and runs through the inversion center). Similarly, the combination of any Cn axis with a vertical mirror plane σv must imply (n - 1) additional vertical mirror planes σv. In addition, combinations of two rotation axes or two rotary-reflection axes must imply a new rotation axis, and combining a rotation axis with a rotary-reflection generates another rotary-reflection axis.
|
|||